|
Background: Moraxella catarrhalis (M. catarrhalis) is a common pathogen in the human upper respiratory tract. This microbe is also implicated in chronic lower respiratory tract infections as well as conjunctivitis, sinusitis, meningitis, otitis media, osteomyelitis, endocarditis, etc. Objectives: This study was carried out to know various facets of M. catarrhalis infection among adults with bronchopulmonary infections and the related antibiotic susceptibility pattern. Materials and Methods: A hospital-based study was carried out among adult participants with history of respiratory tract infection admitted to a tertiary care teaching hospital in Karnataka during the period of May 2007 to April 2010. A total of 912 early morning sputum samples were collected, processed with standard procedures, and analyzed. Results: Out of all the sputum samples, M. catarrhalis was the third most important pathogen (16.01%). Most of these M. catarrhalis isolates were sourced from participants with bronchopneumonia (31.51%), followed by chronic bronchitis (25.34%), bronchiectasis (25.34%), and bronchial asthma (17.81%). M. catarrhalis infection was predominantly noted among males (78.08%) and in older age group (22.60%), i.e., 61-70 years. All strains of M. catarrhalis were sensitive to tetracycline, co-trimoxazole, chloramphenicol, and gentamicin; 75.34% were resistant to penicillin, ampicillin, and amoxycillin. Surprisingly, all strains were resistant to erythromycin; 37 (25.34%) were beta-lactamase positive. Conclusions: M. catarrhalis is one of the emerging pathogens in bronchopulmonary infections, and the beta-lactamase-producing strains imply its ability for antibiotic resistance.
Keywords: Bronchopulmonary infection, beta-lactamase, Moraxella catarrhalis
How to cite this article:
Sirwar SB, Indupalli AS, Pal R, Zaman FA, Kar S. Moraxella catarrhalis: An emerging pathogen in bronchopulmonary infections. Ann Trop Med Public Health 2013;6:76-9 |
How to cite this URL:
Sirwar SB, Indupalli AS, Pal R, Zaman FA, Kar S. Moraxella catarrhalis: An emerging pathogen in bronchopulmonary infections. Ann Trop Med Public Health [serial online] 2013 [cited 2016 Aug 15];6:76-9. Available from: https://www.atmph.org/text.asp?2013/6/1/76/115208 |
Moraxella More Details catarrhalis (M. catarrhalis) is a gram-negative coccus of the Neisseria More Detailse family (synonym: Branhamella catarrhalis). Conventionally, this organism is considered as the respiratory tract commensal, but recent reports suggest this as a pathogenic microbe. [1],[2]
Microbial resistance in respiratory tract infections is a distressing problem for M. catarrhalis. To cope up with this situation, we have to frame intervention strategies from precise regional as well as global epidemiological data. [3]
M. catarrhalis predominantly causes otitis media in the pediatric age groups and lower respiratory tract infections in the aged people; more reports are pouring in as the nosocomial transmission is being progressively more reported and as the risk factor of bacteremia. The extensive creation of a beta-lactamase enzyme helps M. catarrhalis to become resistant to the penicillin. The cephalosporins and beta-lactamase inhibitors are effectual against the beta-lactamase producers, and the organism remains practically susceptible to the common macrolides, fluoroquinolones, and tetracycline. [4]
A fast augmentation in the frequency of beta-lactamase-producing M. catarrhalis isolates had highlighted its pathogenic prospective and requires an uninterrupted monitoring of antibiotic susceptibility in the era of discovery of the BRO beta-lactamase genes. [5]
Nasopharyngeal carriers of M. catarrhalis have been implicated in upper respiratory tract infections and otitis media. [6]
Majority of the published reports of M. catarrhalis were from developed countries. So this study was carried out to find the role of M. catarrhalis in bronchopulmonary infections in adults and its ability to produce beta-lactamase in the isolates and to elucidate its antimicrobial susceptible pattern as well in the Indian participants.
A descriptive cross-sectional hospital-based epidemiological study was carried out among adult participants with provisional diagnoses as obstructive pulmonary diseases and bronchopneumonia admitted to a tertiary care teaching hospital in Karnataka during the period of May 2007 to April 2010. Institutional ethics committee approved the study. All the participants were explained about the study and written informed consent was obtained. A total of 912 early morning sputum samples were collected during the study period in sterile screw cap bottles and were transported to the laboratory without delay. Samples containing less than 10 epithelial cells and more than 25 leukocytes per low-power field were considered for the study.
Sputum samples and cultures were subjected to Gram’s stain; based on Gram’s stain, samples were inoculated on blood agar, MacConkey’s agar, nutrient agar, and chocolate agar and incubated in CO 2 at 37°C for 24-48 hours. Tests such as catalase, oxidase, tube coagulase, phosphatase, bile solubility, insulin fermentation, optochin sensitivity, sugar fermentation, urease, IMViC were done to identify different isolates. M. catarrhalis colonies were identified as suggested by Doern and Morse’s criteria, i.e., gram-negative cocci; oxidase production; growth on 5% sheep blood agar incubated at 37°C in humidified 10% CO 2 ; lack of pigmentation; failure to produce acid from glucose, maltose, and sucrose; and reduction of nitrate to nitrite. All the M. catarrhalis isolates were subjected to antibiotic susceptibility testing by Kirby-Bauer disc diffusion method and were subjected to beta-lactamase test by iodometric method. [7],[8]
The predesigned and pretested semistructured pro forma was used to collect the data on the positive isolates with the related antibiotic susceptibility patterns and was compiled on MS excel spreadsheet to be analyzed subsequently.
A total of 912 early morning sputum samples collected from patients with obstructive pulmonary diseases and bronchopneumonia were processed and analyzed. Out of 912 samples, M. catarrhalis was found to be the third most important pathogen isolated in 146 (16.01%) samples. Other pathogens isolated were Streptococcus pneumoniae (34.98%), Klebsiella pneumoniae (24.01%), and Staphylococcus aureus (9.98%), and M. catarrhalis was isolated as commensals in 15.02% [Table 1].
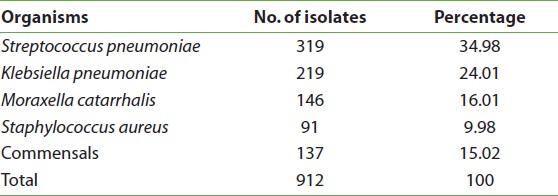 |
Table 1: Different bacterial isolates from the bronchopulmonary infections
Click here to view |
Out of 146 cases of M. catarrhalis, maximum cases (46, 31.51%) were from bronchopneumonia, followed by chronic bronchitis (37, 25.34%), bronchiectasis (37, 25.34%), and bronchial asthma (26, 17.81%). Moraxella infection was predominantly found among 114 (78.08%) males out of 146 and in 33 (22.60%) older age group (i.e., 61-70 years) against 10 (6.85%) younger age group (i.e., 21-30 years).
Of the 146 isolates positive for M. catarrhalis, 37 (25.34%) were beta-lactamase positive. In the antibiogram, all the strains of M. catarrhalis were sensitive to tetracycline, co-trimoxazole, chloramphenicol, and gentamicin, whereas 110 (75.34%) were resistant to penicillin, ampicillin, and amoxycillin. Surprisingly, all strains were resistant to erythromycin [Table 2].
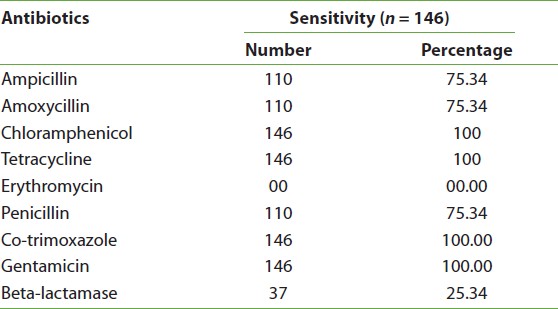 |
Table 2: Antibiotic susceptibility patterns of Moraxella catarrhalis isolates
Click here to view |
Regarding isolates of M. catarrhalis, other researchers in the same field had comparable observations in the range of 1.8-13.2%. [8],[9],[10] In this study, M. catarrhalis was the third important pathogen isolated from bronchopulmonary infections; similar findings were seen in the study done by other researchers. [11],[12]
Regarding the important pathogens causing bronchopulmonary infection, our finding correlated with that of Ahmed et al. in Saudi Arabia who also found M. catarrhalis as the third most important pathogen, but H. influenzae was a primary pathogen in their findings. [11] H. influenzae was not isolated in our work. Sorubbi et al. noted M. catarrhalis to be the second common pathogen next to H. influenzae. [13] Pollard and colleagues in a chest hospital found M. catarrhalis to be the fourth most commonly identified respiratory pathogen. [10]
The male preponderance in our study was comparable to that of the work done by Ahmed et al. [11] Similarly, our observation of the high prevalence of M. catarrhalis infection in the elderly age group coincided with the findings of Verghese et al. [14]
The data of our study showing beta-lactamase positivity indicating antibiotic resistance were close to the data reported by Slevin et al. who observed as 40% and Murphy et al as 10%. [15],[16]
All the strains of M. catarrhalis in our study were sensitive to tetracycline, co-trimoxazole, chloramphenicol, and gentamicin; 25% of strains were resistant to penicillin and ampicillin. Our findings were consistent with those of other investigators. [6],[17],[18]
There was one exceptional finding in our observation that all M. catarrhalis strains were resistant to erythromycin (100%). Comparable pattern of emergence of erythromycin resistance was first reported in 1983. Subsequently, erythromycin resistance was also reported in studies done by others. [16],[19]
In the Polish study, majority of the M. catarrhalis isolates (71%) were resistant to ampicillin; most of these isolates were susceptible to cefaclor (99%), cefuroxime (94%), cefotaxime (100%), ciprofloxacin (100%), tetracycline (91%), co-trimoxazole (93%), combination of amoxycillin and clavulanic acid (100%), and erythromycin (93%). [19]
The strength of our study was that we dealt with an emerging infection of M. catarrhalis that probably causes bronchopulmonary diseases more frequently than appreciated as our conventional teaching-learning in the medical schools. Beta-lactamase-producing strains of M. catarrhalis were detected, indicating that beta-lactamase produced by M. catarrhalis may inactivate the antibiotics such as penicillin, ampicillin, or amoxycillin and protect the pathogens resulting in therapeutic failure of such respiratory infections in the clinical settings.
We had several limitations. The focus of our study was limited only to bronchopulmonary diseases by M. catarrhalis. We should have collected data on the nosocomial transmission and bacteremia associated with M. catarrhalis.
Future directions of our study decidedly suggested the need for similar works to be carried out from different parts of developing countries, particularly in different states of India, to understand the role of M. catarrhalis as an important emerging pathogen. The entirety of M. catarrhalis isolates resistant to erythromycin noted in our study indicated for further evaluation. We also have to explore the wide geographical variations and its ability to produce antibiotic resistance resulting in therapeutic failure in the future.
To sum up, the results of our series indicated that M. catarrhalis has emerged as an important pathogen in respiratory diseases in this geographical area and direct us toward advanced studies to find the other morbidities caused by this microbe.
The authors greatly acknowledge the management and staff of KBN Medical College and Hospital, Gulbarga, Karnataka, for kind cooperation.
| 1. |
Doern GV, Tuber RA. Detection of beta lactamase activity among clinical isolates of Branhamella catarrhalis with six different beta lactamase assays. J Clin Microbiol 1987;25:1380-3. |
| 2. |
Mcleod DT, Ahmed F, Margaret JT. Bronchopulmonary infection due to Br catarrhalis. BMJ 1983;87:144-7. |
| 3. |
Felmingham D, Feldman C, Hryniewicz W, Klugman K, Kohno S, Low DE, et al. Surveillance of resistance in bacteria causing community-acquired respiratory tract infections. Clin Microbiol Infect 2002;8 Suppl 2:12-42. |
| 4. |
McGregor K, Chang BJ, Mee BJ, Riley TV. Moraxella catarrhalis: Clinical significance, antimicrobial susceptibility and BRO beta-lactamases. Eur J Clin Microbiol Infect Dis 1998;17:219-34. |
| 5. |
Köseoglu O, Ergin A, Hascelik G. Evaluation of restriction endonuclease analysis of BRO beta-lactamases in clinical and carrier isolates of Moraxella catarrhalis. Scand J Infect Dis 2004;36:431-4. |
| 6. |
Köseoðlu O, Ergin A, Gürkan Aydin N, Hasçelik G. [Molecular characterization of BRO beta-lactamases of Moraxella catarrhalis strains isolated from carrier children]. Mikrobiyol Bul 2004;38:335-40. |
| 7. |
Mackie, McCartney. Tests for identification of bacteria. Practical Medical Microbiology. In: Collee JG, editor. 14 th ed. New York: Churchill Livingstone; 1996. p. 131-49. |
| 8. |
Doern GV, Morse SA. Branhamella (Neisseria) catarrhalis criteria for lab identification. J Clin Microbiol 1980;11:193-5. |
| 9. |
Jakubicz P, Leszenska K. Occurrence of Moraxella catarrhalis in patients with respiratory tract infection. Med Dosw Microbial 1997;49:55-60. |
| 10. |
Pollord JA, Wallace RJ, Nash DR, Luman JI. Incidence of Br catarrhalis in the sputam of patients with chronic lung disease. Drugs 1986;31:103-8. |
| 11. |
Ahmed S. Bronchopulmonary infection due to Moraxella catarrhalis at a specialist hospital in Saudi Arabia. J Commun Dis 1998;30:233-6. |
| 12. |
Tamang MD, Dey S, Makaju RK, Jha BK, Shivananda PG, Bhramadatan KN. Prevalence of Moraxella catarrhalis, infections of the lower respiratory tract in elderly patients. Kathmandu Univ Med J (KUMJ) 2005;3:39-44. |
| 13. |
Sorubbi A, Myers JW, Jennifer J, Williams BS, Shell BS. Respiratory infection caused by Branhamella catarrhalis selected Epidemiology features. Am J Med 1990;88:5-13. |
| 14. |
Verghese A, Berk SL. Normal throat flora causing pneumonia in your patients. Am Rev Respir Dis 1982;125:783-4. |
| 15. |
Slevin NJ, Aitken J, Thornley PE. Clinical and microbiological features of Br catarrhalis in bronchopulmanary infections. Lancet 1984;1:782-3. |
| 16. |
Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: Burden of disease and immune response. Am J Respir Crit Care Med 2005;172:195-9. |
| 17. |
Stobbringh EB, Davies B, Boren CP. Branhamella catarrhalis antibiotic sensitivity and beta lactamase. J Antimicrob Chemother 1984;13:55-64. |
| 18. |
Fung CP, Powell M, Seymour A, Yuan M, Williams JP. The antimicrobial susceptibility of Moraxella catarrhalis isolated in England and Scotland in 1991. J Antimicrob Chemother 1992;30:47-50. |
| 19. |
Leszczyñska K, Jakoniuk P, Sacha PT, Zalewska M, Wieczorek P. [Susceptibility of Branhamella catarrhalis to antibiotics]. Med Dosw Mikrobiol 2004;56:231-7. |
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1755-6783.115208
[Table 1], [Table 2] |