Objective: The purpose of this study was to compare results obtained using a single fecal specimen for ova and parasite (O and P) examination, direct immunofluorescent assay (DFA), and two conventional staining methods. Design: Fecal specimens of 150 children were collected and examined by each method. The O and P and the DFA were used as the reference methods. Setting: The study was performed at the laboratory in the Basic Medical Science Institute JPMC Karachi. Materials and Methods: The fecal specimens were collected from children with a suspected Giardia lamblia infection. Agreement and disagreement between the methods was determined based on (1) the presence of giardiasis in our population and (2) the sensitivity and specificity of each method. Results: There was 45 (30%) positive and 105 (70%) negative cases found with DFA, 41 (27.4%) positive and 109 (72.6%) negative cases detected by iodine method, and 34 (22.6%) positive and 116 (77.4%) negative cases found with saline method. The sensitivity and specificity of DFA in comparison to iodine were 92.2 and 92.7%, respectively. The sensitivity and specificity of DFA in comparison to saline method were 91.2 and 87.9%, respectively. The sensitivities of iodine method and saline method in comparison to DFA were 82.2 and 68.8%, respectively. There is marked difference in sensitivity of DFA compared to conventional methods. Conclusion: The study supported the findings of other investigators who concluded that DFA method has the greatest sensitivity. The immunologic methods were more efficient and quicker than the conventional O and P method. Keywords: Detection, feces, Giardia lamblia, giardiasis, immunofluorescence, microorganisms
The intestinal protozoon, Giardia, the causative agent of giardiasis, was first described over 300 years ago by Leeuwenhoek. Giardia lamblia is a flagellated protozoon that infects the small intestines of humans and is a major cause of intestinal infection throughout the world. [1] Prevalence rates in children in developed countries are quoted as 2-5% but is up to 20-30% in the developing countries. [2] It is much more common in children than in adults, especially in children under 10 years of age, the prevalence is 2-7 times greater than in adults. [3] The Giardia trophozoite exhibits a characteristic pear, or teardrop, shape with bilateral symmetry when viewed from the top. It is typically 12-15 μm long, 5-10 μm wide, and 2-4 μm thick. Characteristic features of the stained trophozoite include two nuclei with central karyosomes, fibrils running the length of the parasite, and median bodies. The large karyosome and lack of peripheral chromatin gives the nuclei the appearance of a halo. The fibrils are called axonemes and are formed from the proximal regions of the flagella within the body of the trophozoite. The median bodies are a pair of curved rod-shaped structures, which lie posterior to the nuclei. At the ultrastructural level, the median bodies contain an array of microtubules. The functions of the median bodies are not known, but most believe that they are somehow involved with the adhesive disk and its formation. An adhesive disk, not always visible by light microscopy, occupies the ventral side of the anterior end. The Giardia cyst has four nuclei usually located at the anterior end of the cyst. The flagella and adhesive disk are lost as the cyst matures, but the axonemes and median bodies persist. The distinctive fibrils (i.e., axonemes) extend across the length of the cyst. [4] There is no “gold standard” for the detection of G. lamblia. Stool examination is the traditional, safest and easiest method. [5] The initial method of diagnosis is by demonstration of the trophozoite or cysts of G. lamblia in the stool by microscopy or stool antigen detection by enzyme-linked immunosorbent assay (ELISA). Other methods of diagnosis include examination of duodenal contents by aspiration or biopsy with endoscopies. A definitive diagnosis may require repeated stool examinations, fecal immunoassays, or even sampling of the upper intestinal contents. [6] Direct investigations involving stool examination by indirect fluorescent antibody (IFA) or ELISA are also an effective means of diagnosis. [2] Serology and stool culture are generally unnecessary. Polymerase chain reaction (PCR) analysis, while only experimental, may be effective for screening water supplies. [7] The fluorescence stating techniques proved more sensitive than other tests routinely used for diagnosis. [8] The direct immunofluorescent-monoclonal antibodies method resulted in a significantly increased detection rate of Giardia.[9] Furthermore, the monoclonal antibody reagents offer increased sensitivity and an excellent alternative to conventional staining methods. These reagents are helpful when screening large number of patients or those with minimal symptoms. Problem of false-positive and false-negative results with routine staining methods for stool parasites can be eliminated with monoclonal antibody reagents. [10] Giardia direct immunofluorescence test (MERIFLUOR® ) showed 100% sensitivity and specificity. Comparison of two different ELISAs showed sensitivities of 92 and 87% and specificities of 87% and 91%, respectively. [11] Effective means of protection against giardiasis exist and include good hand hygiene, avoiding ingestion of surface water, and preferably drinking only bottled water, or when bottled water is not available, disinfecting/filtering the drinking water. Such advice should be followed by persons traveling to areas with less than optimal hygienic conditions. Special attention, before and after travel, should be given to parents of young children. [12] Nitroimidazoles: The nitroimidazole class of agents used to treat G. lamblia infection includes metronidazole, tinidazole, ornidazole, and secnidazole. Metronidazol (-hydroxyethyl)-2-methyl-5-nitroimidazole). [13]
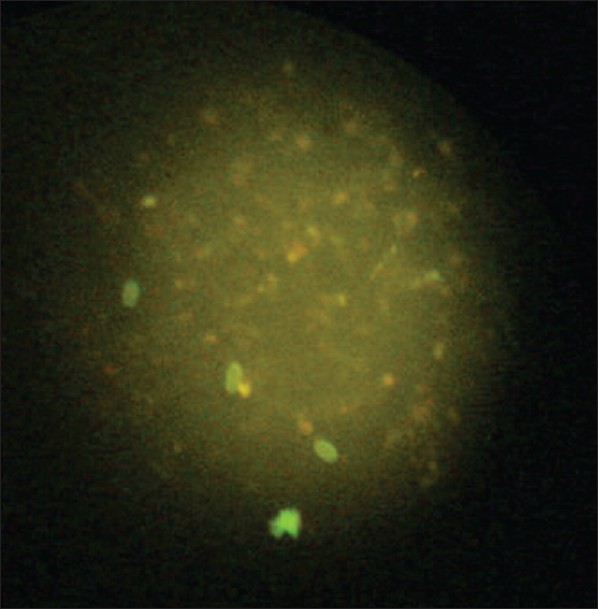
One hundred and fifty fecal specimens from children with suspected G. lamblia infection were collected for Giardia testing. Each specimen was coded and processed separately to eliminate the possibility of observer bias. Specimens were collected in a 10% formalin vial to perform the direct wet mount. The traditional ova and parasite (O and P) method was performed with the specimen from the 10% formalin vial and included microscopic examination of saline and iodine direct wet mount. [14] Testing by immunological method included product from manufacturer (waterborne™). [15] The ST102R.Girdi-a-Glo assay utilized the principle of direct immunofluorescence (DFA). The “detection reagent” contained a mixture of labeled monoclonal antibodies directed against cell wall antigens of Giardia cysts. A smear of the fecal specimen was treated with the detection reagent and then counterstained. The slides were rinsed to remove unbound antibodies, a cover slip was mounted, and the slides were examined for fluorescent apple green color and characteristic morphology of Giardia cysts using a fluorescent microscope [Figure 1].
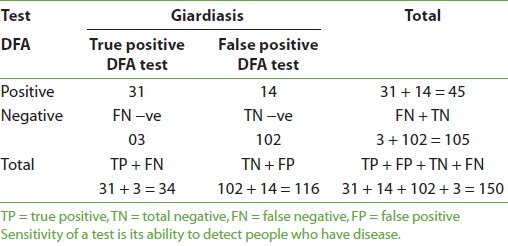
Giardiasis was positive in 45 (30%) and negative in 105 (70%) cases when DFA was used; on using iodine, there were 41 (27.4%) positive, 109 (72.6%) negative cases, and with saline method 34 (22.6%) positive and 116 (77.4%) negative cases were identified. More number of males were found to be positive than females by all three methods. Also, the highest number of positive cases was found in the age group 1-5 years in all three methods. The mean age was found to be 4-5 years by all three methods. The sensitivity of DFA method in comparison to iodine method for giardiasis is shown in [Table 1] and [Figure 1]. The sensitivity was 90.2%, specificity was 92.7%, positive predictive value (PPV) was 82.2%, and negative predictive value (NPV) was 96.2%. There were 8 (5.3%) false-positive and 4 (2.6%) false-negative cases with DFA in comparison to iodine. The sensitivity and specificity of DFA in comparison to saline method were 91.2 and 87.9%, respectively [Table 2] and [Figure 2]. PPV was 82.2% and NPV was 92.7%. There were 8 (5.3%) false-positive and 4 (2.6%) false-negative cases in comparison to iodine method. The sensitivity of iodine method and saline method in comparison to DFA were 82.2 and 68.8%, respectively.
In this study, 27.3% were found to be positive with iodine method, 22.6% were positive with saline method and 30% were found positive with DFA, for giardiasis. In a study conducted by Zimmerman and Needham, [16] they found 6.4% positive cases for giradiasis detected by MERIFLUOR DFA. In, Garcia et al. [10] found a prevalence rate of 27.0% with DFA, which is in accordance with this study. In the present study, 27.3% positive cases of giardiasis were found by the conventional method. Shakkoury and Wandy [17] found a prevalence of 78% in children by the conventional method in 2005. The study conducted by Al-Mekhlafi et al,[18] also showed a prevalence rate of 24.9% in children. In epidemiological meaning, the prevalence may not be established because of the limited number of cases in these studies. The prevalence of giradiasis was found to be highest in childhood by Hokelek and Nissen. [19] In the present study, the highest number of positive cases was found in the age group of 1-5 years, in all three methods. This shows that peak age of 4-5 years is more vulnerable to giardiasis. Kazi et al, [20] found peak age of 4 years, which is in agreement with this study. Pennardt [21] reported that giardiasis has a peak rate of 15-20% in children younger than 10 years, which is also in agreement with the results of this study. In this study, DFA microscopy was found to have 90.2% sensitivity and 92.7% specificity in comparison to the iodine method. With saline method, the sensitivity and specificity were 91.2 and 87.9%, respectively. So, the present study is comparable with that of Zimmerman and Needham conducted, [16] who found the diagnostic sensitivity of MERIFLOUR (DFA) to be 100% and specificity to be 99.8% on control cases, while in this study only suspected cases of giardiasis were included. In the present study, if controls would have been taken, then specificity and sensitivity of DFA could be matched with that of Zimmmerman and Needham. [16] Another study conducted by Zell et al, [22] found 100% sensitivity of DFA on positive donor pool. Garcia et al,[10] reported that monoclonal antibody reagents offer increased sensitivity and are an excellent alternative to the conventional staining methods as the problems of false-positive and false-negative results with routine staining methods for stool parasites can be eliminated with monoclonal antibody reagents, i.e., DFA. DFA method, in addition to having excellent specificity, exhibits vastly improved sensitivity over the conventional methods used. These findings supported and supplement the initial experiences of others with this technique. Technologists can become rapidly proficient in the performance of this simple direct immunofluorescence method and allows rapid scanning of stained slides, saving valuable observation time. Additionally, the high quality of reagents results in minimal background autofluorescence or nonspecific staining and enhances the identification of Giardia cyst appearing as bright apple green, which is easy to identify. False-positive and false-negative cases are reported even by experienced microscopists in the conventional method. In this study, it was found that eight false-negative and four false-positive cases resulted by iodine method in comparison to DFA method. There were 14 false-negative and 3 false-positive cases by saline in comparison to DFA method. This shows that DFA is more superior and effective diagnostic tool than saline and iodine methods for identification of Giardia.
In this study, it was found that DFA is superior to conventional methods for detecting giardiasis. Although the test is considerably more expensive than the conventional staining methods, DFA can replace the conventional method for diagnosis of giardiasis in routine laboratory to minimize the false-positive and false-negative cases. The key role of DFA method is its significance in epidemiological and control studies because of its high sensitivity and specificity and minimal time taken for scanning the slides.
Source of Support: None, Conflict of Interest: None
[Figure 1], [Figure 2]
[Table 1], [Table 2] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||