| Abstract |
Thromboembolic disorders are one of the disorders for which the researchers are still in search for a safe and efficient drug. Despite the widespread use of antithrombotic drugs for the prevention and treatment of arterial and venous thrombosis, thromboembolic diseases continue to be a major cause of death and disability worldwide. This shows the researchers’ inefficiency in searching efficacious and safe antithrombotic drugs. The researchers have reached to the basic mechanism of thrombus formation and by interrupting various steps of this mechanism, they can prevent as well as treat thromboembolic disorders. In continuation of Aspirin, now, the researchers are using clopedogrel, Ticlopidine and GpIIb/IIIa inhibitors (Abciximab, Tirofiban and Eptifibatide). Warfarin is an old antithrombotic drug, which is still being used but due to various side effects and drug interactions, they are bound to use newer drugs. Newer antiplatelet drugs include prasugrel, ticagrelor, cangrelor and elinogrel whereas newer thrombin inhibitors are Ximelgatran and Dabigatran. Apixaban and edoxaban are also newer entry in this category as Factor Xa inhibitors. Idrabiotaparinux is an indirect inhibitor of Xa as it accelerates the activity of antithrombin. Moreover, researches and trials for better and safe drugs are going on.
Keywords: Antiplatelet drugs, Antithrombotic drugs, Thrombin inhibitors
| How to cite this article: Sikka P, Bindra V K. Emerging antithrombotic drugs: A review. Ann Trop Med Public Health 2011;4:138-42 |
| How to cite this URL: Sikka P, Bindra V K. Emerging antithrombotic drugs: A review. Ann Trop Med Public Health [serial online] 2011 [cited 2020 Aug 5];4:138-42. Available from: https://www.atmph.org/text.asp?2011/4/2/138/85773 |
| Introduction |
One of the major causes of morbidities and mortality worldwide is thromboembolic disorder. Thrombosis is the process of formation of solid mass in circulation from constituents of flowing blood, and the mass itself is called as thrombus. Hemostatic plugs are the blood clots formed in healthy individuals at the site of bleeding i.e. they are useful as they stop the escape of blood and plasma, whereas thrombi developing in the unruptured blood vessels may be harmful.
Virchow described three primary events which predispose to thrombus formation (Virchow’s triad) : endothelial injury; alteration in flow of blood and hypercoagulability of blood. [1]
Thrombosis can be either arterial or venous. Both arterial and venous are composed of fibrin, platelets and trapped RBC. Arterial thrombi are commonly formed after endothelial injury due to rapidly flowing blood, thus, platelets are abundant and fibrin is relatively sparse in arterial thrombus, whereas venous thrombus is formed due to blood stasis in veins, thus venous thrombus is mainly composed of fibrin and trapped RBC but less platelets. This fact is important to treat the patient effectively. Antithrombotic drugs are mainly of three types: (I) antiplatelet agents; (II) fibrinolytic drugs and (III) anticoagulants. By knowing the nature of thrombus, we can institute effective therapy i.e. arterial thrombus should be treated by antiplatelet agents [2],[3] and venous thrombosis should be treated by anticoagulants mainly. [4],[5]
Despite the widespread use of antithrombotic drugs for the prevention and treatment of arterial and venous thrombosis, thromboembolic diseases continue to be a major cause of death and disability worldwide. That means available drugs are not so much efficacious and sufficient to combat these disorder. [6] Here we are going to review the older drugs in short, their shortcomings i.e. unmet medical needs of currently available antithrombotic therapy and finally the newer drugs, newer targets and opportunities and challenges for them.
| The Unmet Need of Current Antithrombotic Therapy |
Recurrent ischemic events are quite common if patient with previous history of stroke takes aspirin, clopidogrel, ticlopidine or their combinations regularly i.e. at present most widely prescribed antiplatelet agents are not efficient enough to prevent further attacks. This can be either resistance to these drugs or incomplete suppression of platelets by these drugs.
The only oral anticoagulant available in the market for more than half a century i.e. warfarin also tells the same story with other limitations too. It has very slow onset of action for which it is combined with rapidly acting parenteral anticoagulants for first few days. Due to some genetic polymorphism, dose adjustment is needed in almost every individual because of variable metabolism of Warfarin. There is one important problem of multiple drug interactions and more than this is its narrow therapeutic index. Because of this inconvenience, patient compliance is poor on long-term basis.
Combination therapy (>1 antithrombotic agents at a time) has resulted in declining rate of recurrent ischemia but the incidence of bleeding has increased. [6]
| Natural Anticoagulant Mechanisms |
In healthy vasculature, circulating platelets are maintained in an inactive state by nitric oxide and prostacyclin released by endothelial cells lining the blood vessels. Endothelial cells also express ADPase, which degrades ADP released from RBC and activated platelets, thereby preventing further platelet aggregation. [7] PGI 2 opposes action of TXA 2 and thus inhibits platelet aggregation and release. Antithrombin III (a plasma protein) blocks the action of factors XII, XI, IX, X and II. Protein C (a plasma protein) inactivates factor V and VIII not blocked by AT-III; it also enhances the action of t-PA. Also Heparan sulfate (a proteoglycan related to the Heparin) is synthesized by endothelial cells and it enhances the activity of AT-III. [8]
Both hemostasis and thrombosis depend on three general components-the vascular wall, platelets and the coagulation cascade.
Platelets contain two specific types of granules. Alpha granules express adhesion molecule, P selectin, on their membranes and contain factor V, vWF, PDGF, fibrinogen and TGFb, whereas d (delta) granules contain ADP, calcium, 5HT and histamine. [9]
Vascular injury leads to transient vasoconstriction and finally, platelets are exposed to ECM via vWF and undergo three reactions: (I) adhesion and shape change; (II) secretion (release reaction) and (III) aggregation. [9]
Adhesion of platelets to ECM (extracellular matrix) is facilitated by the interaction of vWF (from endothelium) and Gp IB receptors (on platelets). This adhesion leads to change in shape of platelets and there is release of granules (ADP from dense or delta granules). ADP is a potent mediator of platelet aggregation [9] and it also augments further ADP release. Now, TXA 2 is synthesized and released which is also a mediator of platelets aggregation. On one hand, vessel wall disruption augments platelet activation and aggregation, on the other hand, tissue factor initiates coagulation (extrinsic pathway). Phospholipid complexes on platelets activate intrinsic coagulation pathway. Platelet adhesion involves vWF, whereas aggregation involves GP IIb-IIIa receptors on platelets via fibrinogen. [9] Common coagulation pathway finally produces thrombin, which not only converts fibrinogen to fibrin but also activates platelets. These fibrin strands, along with platelets, now form platelet-fibrin mesh [6] as hemostatic plug or thrombus.
Antiplatelet agents can be classified on the basis of their site of action into those that inhibit (i) adhesion (ii) activation (iii) aggregation and (iv) platelet-mediated links with inflammation [6] [Figure 1].
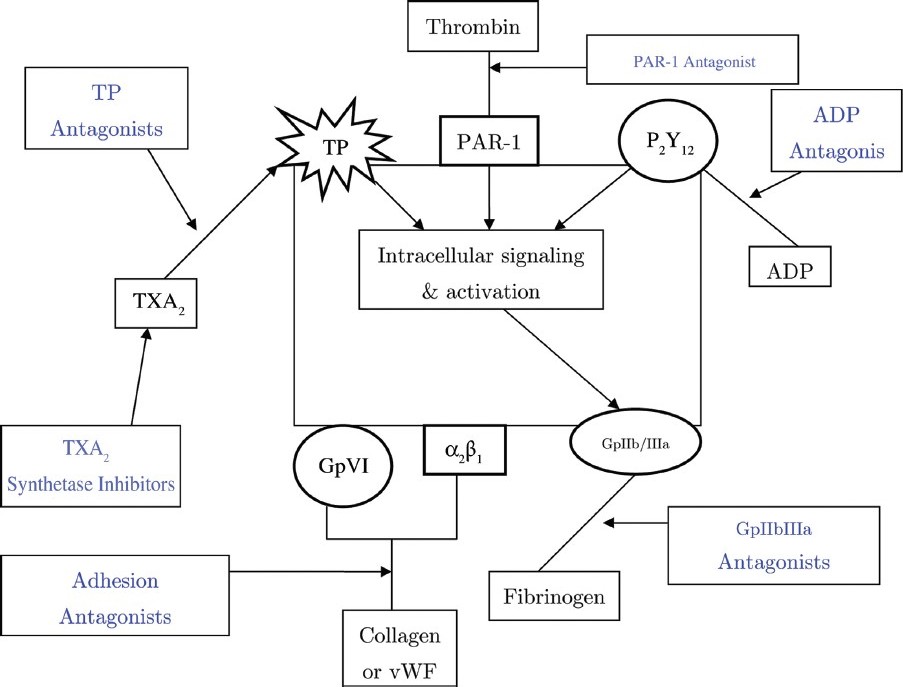 |
Figure 1: Site of action of various antiplatelet drugs: Platelet activation inhibitors include (i) TXA2 pathway inhibitors which block either synthesis or receptors of TXA2, (ii) ADP receptor (P2Y12) inhibitors and (iii) thrombin receptor (PAR-1) inhibitors. Adhesion antagonists block interaction of collagen and vWF with GpVI and α2β1 receptors on platelets. GpIIb/IIIa inhibitors block binding of fi brinogen to their receptors on platelets. ADP, Adenosine di-phosphate; PAR-1, Protease-Activated Receptor-1; TP, Thromboxane receptor; TXA2, Thromboxane A2; vWF, Von Willebrand Factor
Click here to view |
| Inhibitors of Platelet Adhesion |
This step can be inhibited by interfering interaction between GP Ib (platelet receptor) and collagen or vWF. Many drugs are in queue to market which act through above mechanism but none has reached phase III yet. Strategies for inhibiting the ECM-platelet interaction include humanized monoclonal antibodies and aptamers against the receptors, small molecule peptide inhibitors and proteins derived from the medicinal leech. [6]
| Inhibitors of Platelet Activation |
Platelet activation can be inhibited by (i) Inhibiting TXA 2 pathway; (ii) Inhibiting ADP pathway; (iii) Inhibiting thrombin and (iv) Inhibiting PDE.
(i) Inhibitors of TXA 2 pathway : Low-dose (75-325 mg) aspirin inhibits COX-1 in a way that only TXA 2 production is inhibited and not of PGI 2 . GI bleed, drug interactions and resistance are major drawbacks of aspirin, for which newer strategies can be inhibition of thromboxane synthetase and blockade of TXA 2 receptors. [10] TXA 2 synthetase is not much efficacious clinically because blockade at this step results in accumulation of endoperoxide precursors which themselves are platelet agonists. [11]
(ii)Inhibitors of ADP pathway : Clopidogrel and ticlopidine irreversibly inhibit ADP receptors on platelets. Actually, ADP activates ADP receptors (P 2 Y 1 and P 2 Y 12 receptors) to induce shape change and aggregation (P 2 Y 12 effect) and to increase their adhesiveness (P 2 Y 12 effect) . So P 2 Y 1 and P 2 Y 12 inactivation will disfavor platelet aggregation. [12]
However, (i) ceiling effect, (ii) resistance (genetic polymorphism), (iii) drug interactions (drugs metabolized by CYP3A4) and (iv) delayed onset of action are the major drawbacks of Clopidogrel, whereas Ticlopidine has been out marketed because of increased incidence of bleeding and serious neutropenia.
Newer drugs in this category are Prasugrel, Ticagrelor, Cangrelor and Elinogrel. Prasugrel produces more rapid, more consistent and more potent inhibition of ADP-induced platelet aggregation than Clopidogrel does [13] Drug interactions and resistance are not seen with Prasugrel. Onset of action is just 1-2 h. However, it causes more bleeding [14] and should be cautiously given in patients with past history of stroke, age >75 years and weight <60 kg. Ticagrelor, an orally active P 2 Y 12 inhibitor provides more rapid and complete antiplatelet action than Clopidogrel, whereas Cangrelor, a reversible inhibitor of P 2 Y 12 , has rapid onset and offset of action but is administered intravenously. It is still in phase III of clinical trial. Elinogrel is a fast, potent, direct-acting (ie, non-prodrug), selective, competitive, and reversible P 2 Y 12 inhibitor available in both intravenous and oral formulations. They all are in studies for prevention and treatment of STEMI (ST Elevation Myocardial Infarction) and NSTEMI (Non-ST Elevation Myocardial Infarction) following PCI (Per Cutaneous Intervention).
(iii) PAR-I inhibitors : – Protease-activated receptors 1 and 2 are receptors by which thrombin acts for platelet aggregation. PAR-1 antagonists such as SCH530348 and E5555 are under clinical evaluation. [15] SCH530348, an orally active PAR-I inhibitor, is under Phase III and E5555 in undergoing Phase-II evaluation.
(iv) Phosphodiesterase (PDE) inhibitors : – Dipyridamole and Cilostazol are PDE inhibitors. PGI 2 acts through cAMP (as second messenger) thus, more the cAMP, more will be antiplatelet effect but PDE degrades cAMP to form 5-AMP. So, PDE inhibitors tend to retain the amount of cAMP. They are being evaluated for their ability to prevent atherosclerotic diseases.
| Inhibitors of Platelet Aggregation |
Tirofiban, eptifibatide and abciximab are Gp IIb/IIIa inhibitors. Abciximab inhibits not only Gp IIb/IIIa but also a IIb/b3 receptors (for vWF) on platelets, thereby, decreasing aggregation through fibrinogen. [16] It is given i.v. and has shown its efficacy in reducing ischemic events in management of ACS and as adjunctive therapy during PCI; [6] however, trials with orally administered Gp IIb/IIIa inhibitors have failed to demonstrate any benefit. Moreover, a pooled data has shown them to significantly increase mortality in ACS cases. [17] Reason behind this is unknown but may be related to partial agonistic activity or proinflammatory effects. [18] These disappointing results have halted development of this class of drugs. [6]
| Inhibitors of Platelet-dependent Inflammatory Pathway |
Inflammation is an important determinant of progression of atherosclerosis and post-thrombotic syndrome, which complicates DVT [19] through CD40/CD40 ligand pathway and P-selectin. Platelets also play important role in inflammation. [20] CD40 acts as cell attractant molecule and starts proinflammatory response. Also, it is highly expressed in atheromatous plaques. Thus, inhibitors of CD40/CD40 ligand pathway can be novel molecules for delaying progression of atherosclerosis. [21]
P-selectin molecule helps in the formation of platelet leukocyte aggregates. Inhibition of P-selectin will attenuate thrombus stimulus and formation. It has been proved in animal models of DVT [22] but yet to be proved in humans.
| Present Anticoagulants and their Future Prospects |
Oral anticoagulants i.e. warfarin is in use for more than half a century but too much adverse effects push for developments in this field. Drug interactions, very slow onset of action and variable response are major and problematic limitations of warfarin.
Now, we are targeting towards inhibition of (i) thrombin and (ii) other coagulation factors especially Xa.
Thrombin inhibitors
Thrombin is a most potent platelet antagonist; it converts fibrinogen to fibrin and also amplifies its own production. Because of its multiple roles in coagulation, thrombin inhibitors not only block fibrin formation but also attenuate further thrombin formation and platelet activation. [23] The first direct thrombin inhibitor, Ximelgatran has shown its efficacy in atrial fibrillation. The major drawback due to which it has been withdrawn is hepatotoxicity. [24] Latest addition in this series is Dabigatran etexilate. Being a prodrug, it is converted to Dabigatran by esterases after oral administration. T 1/2 is 14-17 h and is excreted out of the kidneys, thus, once or twice daily administration is enough. It is undergoing phase III trial for prevention and treatment of VTE and for stroke prevention in atrial fibrillation. Other trials include comparison between Dabigatran and Warfarin (RE-LY) and two doses of Dabigatran with Warfarin (Re-COVER). Pooled data shows no evidence of hepatotoxicity but Dabigatran level increases when used with P-glycoprotein inhibitors (quinidine, clarithromycin and verapamil). [25],[26]
Factor Xa inhibitors
It has been shown on animal models that upstream inhibition at the level of factor Xa causes less bleeding than downstream blockade of thrombin but experiments in humans have not yet been performed. [27] As a class, high oral bioavailability, rapid onset of action, T 1/2 7-15 h and both renal and extrarenal excretion are few advantages. Rivaroxaban and Apixaban, oral factor Xa inhibitors, are undergoing phase III trial for VTE prophylaxis and after elective hip and knee replacement. Rivaroxaban has been licensed in Europe and Canada for above indications as studies have shown it to be more efficacious and with decreased risk of major bleeding. [28],[29],[30],[31],[32]
Apixaban has shown to be effective for thromboprophylaxis after orthopedic surgery. [33] Edoxaban is a new oral direct factor Xa inhibitor. Raskob et al. evaluated the efficacy and safety of different doses of edoxaban and found it effective for preventing venous thromboembolism after total hip replacement. [34]
Idrabiotaparinux is a unique long-acting pentasaccharide, which acts by binding to antithrombin, thereby causing some conformational change that accelerates the rate at which antithrombin inhibits factor Xa. So, Idrabiotaparinux is an indirect inhibitor of factor Xa. [35] It is given once a week by subcutaneous route.
| Conclusion |
We are using various antithrombotic drugs for more than half a century but not a single one is sufficiently efficacious. If some are efficacious, they produce side effects or delayed onset of action. Every time there is fear of hemorrhage by excessive dose i.e. dose titration and difference in patient to patient response, which pose great problem in practicing these drugs. Moreover, type of thrombus is also important to treat the patient (either venous or arterial) because drugs for different thrombi are different and there should be proper clinical judgment before giving drugs. So, still we are in search of newer and better antithrombotic drugs but there are miles to go.
| References |
| 1. | Harshmohan. Haemodynamic disorders. Text book of pathology, 4 th ed. New Delhi: Jaypee brothers medical publishers; 2000. p 97. |
| 2. | Becker RC, Meade TW, Berger PB, Ezekowitz M, O’Connor CM, Vorchheimer DA, et al. The primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence Based Clinical Practice Guidelines (8 th Edition). Chest 2008;133:776S-814. |
| 3. | Goodman SG, Menon V, Cannon CP, Steg G, Ohman EM, Harrington RA, et al. Acute ST-segment elevation myocardial infarction: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8 th Edition). Chest 2008;133:708S-75. |
| 4. | Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence Based Clinical Practice Guidelines (8 th Edition). Chest 2008;133:381S-453. |
| 5. | Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8 th Edition). Chest 2008;133:454S-545. |
| 6. | Gross PL, Weitz JI. New antithrombotic drugs. Clin Pharmacol Ther 2009;86:139-46. |
| 7. | Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002;8:1227-34. |
| 8. | Sharma HL, Sharma KK. Drugs affecting coagulation, fibrinolysis and platelet functions. Principles of pharmacology, 1 st ed. Hyderabad: Paras medical publishers; 2007. p. 693. |
| 9. | Richard NM. Haemodynamic disorders, Thromboembolic disease and Shock. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic basis of disease, 7 th ed. Pennsylvania: Elsevier publications; 2004. p. 126-7. |
| 10. | Gaussem P, Reny JL, Thalamas C, Chatelain N, Kroumova M, Jude B, et al. The specific thromboxane receptor antagonist S18886: Pharmacokinetic and pharmacodynamic studies. J Thromb Haemost 2005;3:1437-45. |
| 11. | Fitzgerald DJ, Fragetta J, FitzGerald GA. Prostaglandin endoperoxides modulate the response to thromboxane synthase inhibition during coronary thrombosis. J Clin Invest 1988;82:1708-13. |
| 12. | Sharma HL, Sharma KK. Drugs affecting coagulation, fibrinolysis and platelet functions. In: Principles of pharmacology, 1 st ed. Hyderabad: Paras medical publishers; 2007. p. 703. |
| 13. | Jakubowski JA, Matsushima N, Asai F, Naganuma H, Brandt JT, Hirota T, et al. A multiple dose study of prasugrel (CS-747), a novel thienopyridine P2Y12 inhibitor, compared with clopidogrel in healthy humans. Br J Clin Pharmacol 2006;63:421-30. |
| 14. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. |
| 15. | Chackalamannil S, Ahn HS, Xia Y, Doller D, Foster C. Potent Non-Peptide Thrombin Receptor Antagonists. Curr Med Chem Cardiovasc Hematol Agents 2003;1:37-45 . |
| 16. | Sharma HL, Sharma KK. Drugs affecting coagulation, fibrinolysis and platelet functions. Principles of pharmacology, 1 st ed. Hyderabad: Paras medical publishers; 2007. p. 704. |
| 17. | Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: A metanalysis of phase III multicenter randomized trials. Circulation 2001;103:201-6. |
| 18. | Schneider DJ, Taatjes DJ, Sobel BE. Paradoxical inhibition of fibrinogen binding and potentiation of a granule release by specific types of inhibitors of glycoprotein IIb-IIIa. Cardiovasc Res 2000;45:437-46. |
| 19. | Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Tromb Vasc Biol 2008;28:387-91. |
| 20. | Massberg S, Brand K, Grüner S, Page S, Müller E, Müller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 2002;196:887-96. |
| 21. | Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Haematol 2007;14:55-61. |
| 22. | Myers D, Wrobleski S, Londy F, Fex B, Hawley A, Schaub R, et al. New and effective treatment of experimentally induced venous thrombosis with anti-inflammatory rPSGL-Ig. Thromb Haemost 2002;87:374-82. |
| 23. | Weitz JI. Factor Xa or thrombin: Is thrombin a better target? J Thromb Haemost 2007;565-7. |
| 24. | Testa L, Andreotti F, Biondi-Zoccai GG, Burzotta F, Bellocci F, Crea F, et al. Ximelagatran/Melagatran against conventional anticoagulation: A meta-analysis based on 22, 639 patients. Int J Cardiol 2007;122:117-24. |
| 25. | Blech S, Ebner T, Ludwig Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the direct thrombin inhibitor, Dabigatran, in humans. Drug Metab Dispos 2008;36:386-99. |
| 26. | Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47-59. |
| 27. | Furugohri T, Shiozaki Y, Muramatsu S, Honda Y, Matsumoto C, Isobe K, et al. Different antithrombotic properties of factor Xa inhibitor and thrombin inhibitor in rat thrombosis models. Eur J Pharmacol 2005;514:35-42. |
| 28. | Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. |
| 29. | Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: A double-blind, randomised controlled trial. Lancet 2008;372:31-9. |
| 30. | Turpie Alexander GG, Lassen MR, Kakkar AK, Eriksson B, Misselwitz F, Bandel TJ, et al. A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopedic surgery: Effect on symptomatic venous thromboembolism, death and bleeding. Blood 2008;112:36a. |
| 31. | Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86. |
| 32. | Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673-80. |
| 33. | Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007;5:2368-75. |
| 34. | Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 2010;104:633-41. |
| 35. | Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol 2008;28:380-6. |
Source of Support: None, Conflict of Interest: None
| Check |
DOI: 10.4103/1755-6783.85773
| Figures |
[Figure 1]



