Ripening is the final stage of the maturation process, when the fruit changes color, softens and develops the flavor, texture and aroma that constitute optimum eating quality. This study was conducted to discuss the use of unsatisfactory calcium carbide to ripen fruits for domestic markets as well as their toxic effects on human health. The commonly used ripening agents are calcium carbide, acetylene, ethylene, propylene, ethrel (2-chloroethyl phosphonic acid), glycol, ethanol and some other agents. The calcium carbide is one of the most commonly used ripening agent for fruits, while other calcium salts like calcium ammonium nitrate, calcium chloride and calcium sulfate are used to delay fruit ripening agents for local fruit industries. The use of calcium carbide is being discouraged worldwide, due to associated health hazards. Calcium carbide treatment of food is extremely hazardous because it contains traces of arsenic and phosphorous, and once dissolved in water, it produces acetylene gas. Arsenic, phosphorous and acetylene gas may affect the different body organs and causes various health problems like headache, dizziness, mood disturbances, sleepiness, mental confusion, memory loss, cerebral edema, seizures and prolonged hypoxia. Keywords: Calcium carbide, fruit ripening agents, human health, toxic effects
Effects of the fruits on human health Fruits are widely distributed in nature, commercially important and nutritionally indispensable food commodity. Being a part of a balanced diet, fruits play a vital role in human nutrition by supplying the necessary growth regulating factors essential for maintaining normal health. Whereas explode obesity, diabetes, cardiovascular and cancer diseases are in the majority of the areas in the World, the fruits represent a hope potentially very high to limit the harmful effects of them. Nevertheless, much of way remains to be made for better knowing the impact of the fruits and their components, on the health and the prevention of the principal chronic diseases. Beyond the consumable part of the fruits, an emphasis are also put on the by-products, such as the fruit peels, that could represent precious layers for food, medicinal or cosmetic purposes. Other factors that influence their economic value is the relatively short ripening period and reduced post-harvest life. Fruit ripening is a highly coordinated, genetically programmed and an irreversible phenomenon involving a series of physiological, biochemical and organoleptic changes, that finally lead to the development of a soft edible ripe fruit with desirable quality attributes. Excessive textural softening during ripening leads to adverse effects upon storage. Carbohydrates play a major role in the ripening process, by way of depolymerization leading to decreased molecular size with concomitant increase in the levels of ripening inducing specific enzymes, whose target differ from fruit to fruit. [1] The fruit and vegetables may require few days to ripen and this short period seriously limits its commercialization in distant markets. The unripe fruits is collected and they are ripped by using different chemicals as fruit ripening agents. [2] Ripening agents In recent years, there has been considerable research in the literature concerning the action of different chemicals on the ripening processes of fruits. Under certain conditions, it would appear that these chemicals are capable of hastening the ripening of some fruits and vegetables [1] as shown by the rates of softening, respiration, starch hydrolysis, flavor and color changes. The different ripening agents like calcium carbide, acetylene, ethylene, propylene, ethrel (2-chloroethyl phosphonic acid), glycol, ethanol and some other agents are used for ripening of fruits and vegetables. The ethylene glycol, when diluted with water, can ripen various fruits faster than the regular ripening rate of the fruits, in particular colder climatic conditions. Water does not take away the effects of ethylene glycol in the ripening of fruits. [3] Ethanol can potentially be used to localize ripe fruit, and consumption of low-concentration ethanol within fruit may act as a feeding stimulant. Ethanol is a naturally occurring substance resulting from the fermentation by yeast of fruit sugars. [4] Ethylene is a flammable colorless gas with a sweet odor. Ethylene is a naturally occurring plant hormone that is produced by many fruits and vegetables. It affects the physiological processes in plants and initiates the ripening process when internal concentrations increase from 0.1 to 1.0 ppm (parts per million). Externally applied ethylene can also initiate the ripening process. [5] There has been considerable research in the literature concerning the action of acetylene, ethylene and propylene on the ripening processes of fruits in both normal and chilled fruits and vegetables. Under certain conditions, it would appear that both of these gases are capable of hastening the ripening of some fruits, whether the action of these gases is due to the fact that they are unsaturated compounds. [1] The experiments reported that the “carbide treatment” hastens the ripening processes of fruits and vegetables in both favorable and unfavorable conditions such as chilling. [1] These effects are due to acetylene rather than ammonia or some other impurity. Calcium carbide is known to cause cancer and also causes food poisoning, gastric irritation and mouth ulcers. According to scientists, when calcium carbide comes in contact with moisture in the atmosphere, it produces acetylene gas, which like ethylene accelerates the ripening process. Research literature indicates that ethrel/ethephon (2-chloroethylphosphonic acid) and ethanol are the two potential chemicals that can be used to ripen fruits. [6],[7],[8] On application, these chemicals penetrate into the fruit and decompose into ethylene. While there are only few reports about the effectiveness of ethephon as a ripening agent, [9] ethanol (70% ethanol) is being used for ripening fruit. [10] Effect of other calcium salts on fruits On the other hand, fruits and vegetables have poor storage qualities, and technologies for long-term storage such as controlled or modified atmosphere have not been applied successfully to these fruits and vegetables. They stored in modified atmosphere often show undesirable characteristics, i.e. poor color, poor eating quality and presence of undesirable flavors. So, to solve the problem of short shelf-life of fruits and vegetables, different chemicals are used to delay the ripening. [11] Although calcium carbide has been frequently used since long times to enhance ripening process of fruits; [12],[13] however, some other calcium salts especially calcium chloride (CaCl 2 .2H 2 O), calcium sulfate (CaSO 4 .2H 2 O) or calcium ammonium nitrate Ca(NH 4 NO 3 ) 2 have been reported in literature to delay the ripening and senescence in fruits by lowering the respiration rate. [14] The calcium salts in different concentrations have either been used as pre-harvest sprays or infiltrated into harvested fruits, while some workers treated the harvested fruits by immersing in calcium solution for varying times. These calcium salts maintain organoleptic properties (like skin color, skin shriveling, aroma, pulp color, flavor and taste) of the fruits for long duration as compared with control ripening. Therefore, different concentrations of various calcium salts (i.e. calcium chloride, calcium sulfate and calcium ammonium nitrate) were used to ascertain their effects on delaying the ripening and eating quality of fruits. [2]
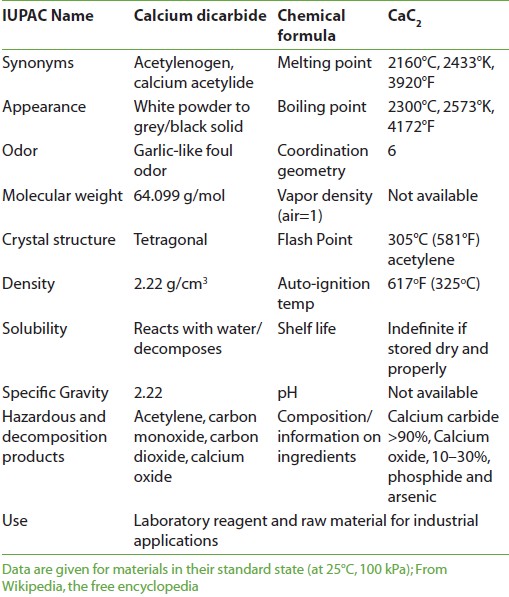
The experiments reported that the “calcium carbide (CaC 2 )” treatment hastens the ripening processes of unripe fruits as shown by the rates of softening, respiration, flavor and color changes. Calcium carbide [Figure 1] is mostly used for ripening of fruit; while, its use is being discouraged worldwide, due to associated health hazards. [10] Secondly, calcium carbide (CaC 2 ) is the commonly used chemical for ripening of fruits, due to its low price and availability in local market; however, use of this chemical in fruit industry is being discouraged worldwide due to dangers of explosion and carryover of toxic materials like arsenic and phosphorus to consumers, thus making the healthy fruit poisonous. [15] Since no technical knowledge is considered necessary for its anomalous use, [16] higher quantity of calcium carbide needed to ripen immature fruit makes them tasteless. [7] In view of the above problems, studies for an alternate ripening agent for fruit were imperative. Further, the local industry is also looking to replace calcium carbide with any suitable alternate. Calcium carbide absorbs moisture and produces acetylene, which is a weak analog of ethylene, responsible for triggering ripening process. [6],[8] Today or in future we will be able find out some better alternatives for fruit ripening agents that have minimal or are without health hazards. Physical and chemical properties of calcium carbide are shown in [Table 1].
Production Calcium carbide (CaC 2 ) is a chemical compound, pure material is colorless, but most samples have a color ranging from black to grayish-white. Its main use industrially is in the production of acetylene and calcium cyanamide. Calcium carbide is produced industrially in an electric arc furnace from a mixture of lime and coke at approximately 2000°C and produced generally around 80% calcium carbide by weight. CaO + 3 C → CaC 2 + CO The CaC 2 content of the product is assayed by measuring the amount of acetylene produced on hydrolysis. Impurities present in the carbide include phosphide, which produces phosphine when hydrolyzed with heavy metals like arsenic. [17],[18],[19],[20],[21],[22],[23],[24],[25] Production of acetylene Calcium carbide (CaC 2 ) is used to make acetylene gas (for use in acetylene torches for welding) and in the manufacturing of plastics (polyvinyl chloride). The reaction of calcium carbide with water was discovered by Friedrich Wohler in 1862. CaC 2 + 2 H 2 O → C 2 H 2 + Ca(OH) 2 This reaction is the basis of the industrial manufacture of acetylene as major industrial use of calcium carbide. Production of calcium cyanamide Calcium carbide reacts with nitrogen at high temperature to form calcium cyanamide and it is used as fertilizer. It is hydrolyzed to cyanamide, H 2 NCN. CaC 2 + N 2 → CaCN 2 + C Steel making Calcium carbide is used in the desulfurization of iron (pig iron, cast iron and steel) as a fuel in steelmaking to extend the scrap ratio to liquid iron and as a powerful deoxidizer at ladle treatment facilities. [17],[18],[19],[20],[21],[22],[23],[24],[25] Carbide lamps Calcium carbide is also used in small carbide lamps called carbide candles, which are used for blackening rifle sights to reduce glare. These “candles” are used due to the sooty flame produced by acetylene. Hazards identification, potential health effects of calcium carbide and first aid measures Calcium carbide is a dangerous and corrosive chemical compound. It causes various side effects on human health. Calcium carbide is harmful to aquatic life at low concentrations. This article is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this product. Individuals receiving the information must exercise their independent judgment in determining its appropriateness for a particular purpose. Transfer promptly to a medical facility. Medical observation is recommended as symptoms may be delayed. Always seek professional medical attention after first aid measures are provided. [17],[18],[19],[20],[21],[22],[23],[24],[25] Eye contact Calcium carbide may cause severe eye irritation with possible burns. Eye contact may result in permanent eye damage or blindness. It may cause stinging pain, severe burns, watering of eyes, inflammation of eyelids and conjunctivitis, opacity and scarring. Protection Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Remove contact lenses if worn. Get medical aid immediately. Wear appropriate protective eyeglasses or chemical safety goggles or face protection regulations. Skin contact Calcium carbide may cause severe skin irritation, rash, burning feeling, redness, with possible burns. Its contact with skin causes irritation and possible burns, especially if the skin is wet or moist. When in contact with moist skin, caustic lime is formed, which can lead to ulceration and scarring. Repeated/prolonged skin contact may cause dryness or rashes. Protection Immediately flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing, shoes and wash contaminated skin with large amounts of water. Wear appropriate gloves to prevent skin exposure. Get medical aid immediately. Ingestion It causes digestive, respiratory and gastrointestinal tract burns, nausea, vomiting, bloating and headache. Protection Rinse mouth with cold water. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Give victim 1-2 tbsp of activated charcoal mixed with 8 oz water. If problem is serious, get medical aid immediately and call Poison Control immediately. Inhalation Mouth, nose, throat and lung irritation with coughing and severe shortness of breath (pulmonary edema), rapid irregular breathing, headache and burns to mucous membranes. May cause severe irritation of the upper respiratory tract with pain, burns, inflammation and can produce delayed pulmonary edema. Repeated inhalation may cause chronic bronchitis. Protection Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Remove the person from exposure. Get medical aid immediately. First aid measures Wear appropriate protective clothing to prevent contact with skin and eyes. Wear a self-contained breathing apparatus (SCBA) to prevent contact with thermal decomposition products. Wear chemical splash goggles and chemical resistant clothing such as aprons and gloves (nitrile and natural rubber). Wash hands thoroughly after handling material and before eating or drinking. Use respirator with a dust cartridge. Contact with moisture or water may generate sufficient heat to ignite nearby combustible materials by formation of flammable acetylene gas. Contact with acid or acid fumes evolves heat and flammable vapors. Begin artificial respiration if breathing has stopped and CPR if necessary. Risk phases or conditions to avoid Incompatible materials, contact with water, acids, exposure to moist air or water, oxidizers. Contact with water liberates highly flammable gases. Contact with water produces heat and flammable acetylene gas and possibly fire. Fire risk when added to water. Fire fighting measures Flammable solid, when heated to decomposition, emits acrid fumes. Contact with water produces flammable acetylene gas which will ignite. Poisonous gases are produced in fire, including calcium oxides. Do not use carbon dioxide, foam, water and halogenated agents. Use approved class D extinguishing agents or smother with dry sand, dry clay or dry ground limestone dry sodium chloride or dry graphite, sodium bicarbonate and dry chemical. Isolation distance Cover with dry lime, sand or soda ash and place into sealed containers for disposal. Use only non-sparking tools and equipment, especially when opening and closing of containers. Handling and storage Use with adequate ventilation and do not breathe dust or vapor. Avoid contact with skin, eyes, or clothing. Minimize dust generation and accumulation. Do not ingest or inhale. Wash hands thoroughly after handling. Store in tightly closed metal containers, in a cool, dry, well ventilated, locked store room away from incompatible materials. Keep away from water, sources of ignition and oxidizing materials. Avoid generating dusty conditions. Use a spark-proof tool especially when opening and closing containers. Store in flammable area with other flammable materials. Stability and reactivity Stable under normal conditions of use like, normal temperatures and pressures. Reacts vigorously with water to form highly flammable acetylene gas, which may ignite spontaneously. Lime may also be formed raising the pH of the solution and causing a white precipitate. Toxicology information Material has not been found to be a carcinogen nor produce genetic, reproductive, or developmental effects. Reactivity Calcium carbide reacts with water and moisture to produce flammable acetylene gas and lime. The heat of the reaction may ignite the acetylene. It reacts with copper, silver, mercury and brass to form explosive compounds such as metal acetylide. Incompatibility Calcium carbide is not compatible with methanol; oxidizing agents (such as per chlorates, peroxides, permanganates, chlorates, nitrates, chlorine, bromine and fluorine); strong acids (such as hydrochloric, sulfuric and nitric); acid fumes; strong bases (such as sodium hydroxide and potassium hydroxide); and metal salts and metal oxides (such as iron chloride and iron oxide). Incompatible with water or moisture, unalloyed copper, silver and mercury, lead fluoride, magnesium, selenium, silver nitrate, sulfur, silver nitrate, sodium peroxide, stannous chloride and chlorine. Disposal considerations Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate disposal considerations. Check with all applicable local, regional, and national laws and regulations. Local regulations may be more stringent than regional or national regulations. Use a licensed chemical waste disposal firm for proper disposal. [17],[18],[19],[20],[21],[22],[23],[24],[25] Punishment for artificial ripening It would not be easy for fruit vendors to ripen fruits artificially now as the Union Health Ministry has decided to punish guilty vendors with up to six months in jail and fine of Rs. 1000. Experts stated that calcium carbide is often used by fruit vendors to ripen fruits like apples, mangoes, bananas, papayas etc. State authorities have been asked by the ministry to keep a strict vigil on use of carbide gas for ripening fruits. Under Rule 44 AA of the Prevention of Food Adulteration (PFA) Rules, 1955, it prohibits use of such chemicals. A circular to state food authorities has been sent by the ministry with the Food Safety and Standards Authority of India (FSSAI) pressing the need to take legal action for violation of the PFA rules. The copy of procedures for detection of acetylene has been sent by the FSSAI to godowns or treatment chambers. The use of such harmful chemicals to ripen fruits faster can cause cancer. [26] General information and effect of arsenic metal Heavy metal toxicity represents an uncommon, yet clinically significant, medical condition. If unrecognized or inappropriately treated, heavy metal toxicity can result in significant morbidity and mortality. Many metals are essential to biochemical processes, and uses in medicine. The pathophysiology of the heavy metals remains relatively constant. The heavy metals bind to oxygen, nitrogen, and sulfhydryl groups in proteins, resulting in alterations of enzymatic activity. This affinity of metal species for sulfhydryl groups serves a protective role in heavy metal homeostasis as well. Increased synthesis of metal binding proteins in response to elevated levels of a number of metals is the body΄s primary defense against poisoning. Nearly all organ systems are involved in heavy metal toxicity; however, the most commonly involved organ systems include the center nervous system, peripheral nervous system, gastrointestinal, hematopoietic, renal, and cardiovascular. The organ systems affected and the severity of the toxicity vary with the particular heavy metal involved, the age of the individual, and the level of toxicity. Generally, children are more susceptible to the toxic effects of the heavy metals and are more prone to accidental exposures. [27],[28],[29],[30],[31] Arsenic is a naturally occurring element widely distributed in the earth’s crust. It combines with oxygen and other elements to form inorganic arsenic compounds. It is used in the formulation of pesticides and fungicides and also manufacturing of glass. Inorganic arsenic compounds also can be used in pressure-treated wood. Inorganic arsenic compounds can dissolve in water, get into food, or blow on the wind in arsenic-containing soil. Arsenic can get into plants when their roots take up water that contains arsenic. It can get into animals when they eat food, drink water, or breathe air that contains arsenic. In plants and animals, arsenic combines with carbon and hydrogen to form organic arsenic compounds. Organic arsenic compounds are less toxic than inorganic arsenic compounds. Arsenic can build up in fish and shellfish, but the arsenic in fish is mostly the organic form and therefore much less harmful. Health effects People exposed to high levels of arsenic can have nausea and vomiting, diarrhea, anemia, and low blood pressure. These symptoms may be followed by a feeling of “pins and needles” in the hands and feet (neuropathy). Chronic (long-term) exposure to arsenic can cause stomach ailments, headaches, fatigue, neuropathy, dark splotches on the skin, and small “corns” or “warts” on the palms of the hands, soles of the feet, and torso. People exposed to inorganic arsenic can have more cancer of the lung, skin, bladder, liver, kidney, and prostate. Studies have not linked arsenic exposure to leukemia in adults or children. Normal urine levels of arsenic are less than 50 μg/L. A level between 50 and 200 μg/L not necessarily represent a health risk. A level over 200 μg/L is considered abnormal and may require treatment if symptoms of arsenic poisoning are present. Intentional or unintentional ingestion of arsenic has been notorious as a means of suicide and homicide. Arsenic is used in rodenticide. Arsenic exposure produced severe edema of the eyelids, gastrointestinal irritation, and both central and peripheral neuropathies. It is the first antidote to heavy metal poisoning, and the basis for chelation therapy today. British Anti-Lewisite (dimercaprol) has sulfhydryl groups that bind arsenic, as well as other metals, to form stable covalent bonds in a process called complexation. The nonionic complexes can then be excreted by the body. Although a high level of suspicion for arsenic poisoning must be maintained because of its role in poisoning, it is rarely seen clinically. [27],[28],[29],[30],[31] Physical findings in arsenic toxicity vary with age and dose. Any combination of GI complaints, neurologic dysfunction, and anemia should prompt a search for arsenic toxicity. GI complaints predominate in adults. Children are more prone to CNS dysfunction, including encephalopathy. Encephalopathy is rare in adults. Encephalopathy may present as an acute event with seizures, or it may develop slowly over weeks to months with variable nonspecific complaints. Cholera like diarrhea can be seen in acute arsenic toxicity. Neurologic complaints ranging from neuropathy to encephalopathy have been reported in cases of acute arsenic toxicity. Arsenic toxicity presenting as ascending flaccid paralysis is often. Medication The key to treating arsenic toxicity is removal of the offending agent and reducing the total body load of arsenic. Chelation agents are used to reduce the body stores of arsenic. Arsenic toxicity Chelation therapy with Dimercaprol (British Anti-Lewisite; BAL)-DOC, DMSA, or d-penicillamine and edetate calcium disodium is the primary treatment of arsenic or other metal toxicity. Removal of the offending agent and aggressive gastric decontamination aids in reducing ongoing absorption of arsenic. Hemodialysis may be beneficial in patients with acute renal failure. Precautions and contraindication May be nephrotoxic and may cause hypertension; caution with oliguria or G-6-PD deficiency; may induce hemolysis in G-6-PD deficiency. Contraindications with hypersensitivity, renal insufficiency, previous penicillamine-related aplastic anemia. Care must be taken to remove the source of heavy metal contamination. [27],[28],[29],[30],[31] General information and effect of phosphorus toxicity Phosphorus is pentavalent in combination with oxygen, as phosphate (PO4 3- ). The toxicity of phosphorus in humans focus on accidental or intentional ingestion of the more toxic forms of phosphorus that are not found in food or food supplements (elemental yellow phosphorus). No human data on chronic toxicity of dietary forms of phosphorus were identified in the literature. The predominant adverse reaction to orally administered phosphorus (as various phosphate salts) in human supplementation studies is osmotic diarrhea, which has been reported at intakes of 750 mg/day and above. Other mild gastrointestinal effects, including nausea and vomiting have been noted in some studies. There are a limited number of studies on the oral toxicity of phosphate salts in laboratory animals. Kidney lesions have been reported in rats by acute doses of phosphates. Pathological effects in the parathyroids, kidneys and bone have also been reported in subchronic studies at high doses. No adverse effects on growth and reproduction were reported. No data on carcinogenicity or genotoxicity of dietary forms of inorganic phosphorus and phosphate salts were identified. One study suggested that increased phosphorus intake may lead to increased bone resorption in postmenopausal women with osteoporosis. However, a study in postmenopausal women found no biochemical evidence of increased bone remodelling as a result of supplementation with phosphorus. Black and Asian people and older people may be susceptible to bone resorption as a result of high phosphorus intakes, as they are more susceptible to hypovitaminosis D, which decreases the absorption of calcium, and phosphorus has been shown to influence the parathyroid-vitamin D axis, causing an increase in serum calcium levels via bone resorption. [32],[33],[34]
The fast ripening of fruits means they may contain various harmful properties. A commonly used agent in the ripening process is calcium carbide, a material most commonly used for welding purposes. Calcium carbide treatment of food is extremely hazardous because it contains traces of heavy metal arsenic and phosphorous. The calcium carbide produces acetylene gas when react with water. Acetylene gas may affect the neurological system by inducing prolonged hypoxia. Calcium carbide causes various health hazards like, headache, dizziness, mood disturbances, sleepiness, mental confusion, memory loss, cerebral edema and seizures. These results indicate that fruit treatment with calcium salts not only affects the ripening process but also influences the aroma of the fruits. The commonly used ripening agents other than calcium carbide are acetylene, ethylene, propylene, ethrel (2-chloroethyl phosphonic acid), glycol, ethanol and some other agents. [35],[36] The calcium carbide is one of the most commonly used ripening agent for fruits while other calcium salts like calcium ammonium nitrate, calcium chloride and calcium sulphate are used to delay fruit ripening agents for local fruit industries.
Source of Support: None, Conflict of Interest: None
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||