|
Context: Cysticercosis is quite common in the tropics. Fine needle aspiration cytology (FNAC) plays an important role in prompt diagnosis of this disease. Aims: The aim of this study was to study the role of FNAC in the diagnosis of cysticercosis. Materials and Methods: Among all the subcutaneous swellings referred for FNAC to our tertiary care hospital during the time period from 2009 to 2011, we found thirty cases of cysticercus infestation which were clinically diagnosed as tuberculous lymphadenitis, reactive lymphadenitis and lipoma. We also reviewed all the reported subcutaneous swellings during that period, which were already classified as acute suppurative processes (forty), for the presence of any parasite fragments. Results: In twenty-eight cases, a definitive diagnosis of cysticercosis was obtained in the form of fragments of parasite bladder wall, and biopsy confirmed the diagnosis in three cases. Two of the forty cases, which were initially reported as acute suppurative lesions during routine reporting, were retrospectively reviewed and parasite fragments were observed. Remaining 38 cases were extensively searched for any evidence of the parasite: however, they only showed acute suppurative inflammation with eosinophils, neutrophils and histiocytes. Conclusions: FNAC for diagnosis of cysticercosis is a low-cost, outpatient procedure. The cytological diagnosis is quite clear where the actual parasitic structures are seen in the smears. However, in other cases, the presence of eosinophils, histiocytes, and a typical granular dirty background should always alert the pathologist to the possibility of this condition. In endemic areas, it should be considered as one of the differential diagnoses for all subcutaneous swellings.
Keywords: Cysticercosis; fine needle aspiration cytology; parasite
How to cite this article:
Kodiatte T, Chinaiah P, Mothakapalli T, Kumar H. Cysticercus cellulosae lies in the eyes of the beholder. Ann Trop Med Public Health 2013;6:201-5 |
How to cite this URL:
Kodiatte T, Chinaiah P, Mothakapalli T, Kumar H. Cysticercus cellulosae lies in the eyes of the beholder. Ann Trop Med Public Health [serial online] 2013 [cited 2020 Dec 2];6:201-5. Available from: https://www.atmph.org/text.asp?2013/6/2/201/116522 |
Cysticercosis is the most common parasitic disease worldwide with an estimated prevalence of greater than 50 million persons. It is endemic in Mexico, Central and South America, and parts of Africa, Asia, and India. [1] Taeniasis is common in the Indian subcontinent because of poor hygiene conditions, frequent consumption of poorly cooked meat and vegetables, and unclean pet animals. [2] Human cysticercosis commonly manifests as subcutaneous and intramuscular nodules. [3],[4],[5] Fine needle aspiration cytology (FNAC) is now available as a preoperative tool for the diagnosis of subcutaneous cysticercosis. [3] The aim of this study was to highlight the importance of the cytomorphology of cysticercus in assessing subcutaneous and intramuscular lesions in endemic areas like India, with a high suspicion in acute suppurative lesions, by a thorough search for the parasitic fragment.
Among all the subcutaneous swellings referred for FNAC to our tertiary care hospital during the time period from 2009 to 2011, we found thirty cases of cysticercus infestation. Palpable subcutaneous and intramuscular nodules were seen at different sites, which were clinically diagnosed as tuberculous lymphadenitis, reactive lymphadenitis, lipoma, neurofibroma, sialadenitis, abscess and secondaries. During the same time period, all the subcutaneous swellings that were already reported as acute suppurative processes (forty) were reviewed again for any parasite fragment. Swellings in the breast and thyroid were excluded from the study. Aspirations were performed with a 23 gauge needle and a 10 ml disposable syringe. Material obtained was smeared onto glass slides, most of which were fixed immediately in 95% methanol and stained with two stains-Haematoxylin and Eosin stain and Papanicolaou stain. One air-dried smear was stained with Giemsa stain.
This study included thirty patients in the age group from 5 to 68 years with a mean age of 25.77 ± 20.81 years. Fifteen patients in the present study were males and fifteen were females. Maximum number of cases [Table 1] were seen in the 1 st decade of life, (30%) followed by 2 nd decade of life (26.67%). and then the 5 th decade (16.67%). With regards to location of lesion [Table 2], maximum cases were seen in the head and neck region (57%), followed by chest (17%) and upper extremities (13%). Clinical diagnosis [Table 3] of tuberculous lymphadenitis was given for 13 cases, lipoma for 8 cases and neuroma in 3 cases. Other provisional clinical diagnoses given were neurofibroma, fibroma, sialadenitis, and secondaries. In twenty-nine cases, aspiration yielded a few drops of clear, pearly white fluid with chalky membranous pieces which were difficult to spread on the slide [Figure 1]. Remaining one case yielded purulent material.In twenty-eight cases, a definitive diagnosis of cysticercosis was obtained in the form of fragments of larval parenchymal wall, and, the biopsy confirmed the diagnosis in three cases.The smears in all the aspirates consisted of a mixed inflammatory cell population composed of neutrophils, eosinophils and lymphocytes. Palisading histiocytes and foreign body giant cells were also seen.The parenchymatous fragments consisted of a reticulum of loose fibrillary stroma, mesenchymal fibers with multiple granular parasitic nuclei and calcareous corpuscles interspersed in it [Figure 2]. One case showed hooklet of cysticercus. This was characteristically sickle-shaped having a refractile-curved portion (blade) with a pointed end and non-refractile bifurcated blunt ends [Figure 3]. Out of the forty acute suppurative lesions that were retrospectively screened in this study, two cases had parasite fragments. Remaining thirty-eight cases were extensively searched for evidence of parasite, but only showed acute suppurative inflammation with eosinophils, neutrophils and histiocytes.Histology was available in three cases for which FNA was done prior to excision of the nodule. Larval Taenia solium cyst was seen in the sections of the lesion. The larva with its three layers (outer cuticle layer, middle nuclear layer and inner parenchymal layer) was identified. An entire cysticercus was seen within the bladder walls within which was seen the scolex [Figure 4]. The extensive folding of the spiral canal and one sucker of the scolex were apparent. Calcareous corpuscles were seen in the fibrous tissues. Hooklets were also observed in these cases [Figure 4].
|
Figure 1: Showing gross aspirate with clear, pearly white fluid containing chalky membranous pieces which were difficult to spread on the slide
Click here to view |
|
Figure 2: Showing microphotographs of the cytomorphology of cysticercus: 2a, b, d: May GrunwaldGiemsa Stain: Cysticercus wall fragments (40×, 100×) 2c : May Grunwald Giemsa Stain: The parenchymatous fragments consisted of reticulum of loose fibrillary stroma with multiple granular parasitic nuclei and calcospherules interspersed in it (400×)
Click here to view |
|
Figure 3: Showing microphotograph of Hooklet (May GrunwaldGiemsa Stain): 100× with 400× inset-Hooklets are sickle-shaped having a refractile pointed end and non-refractile, bifurcated blunt ends
Click here to view |
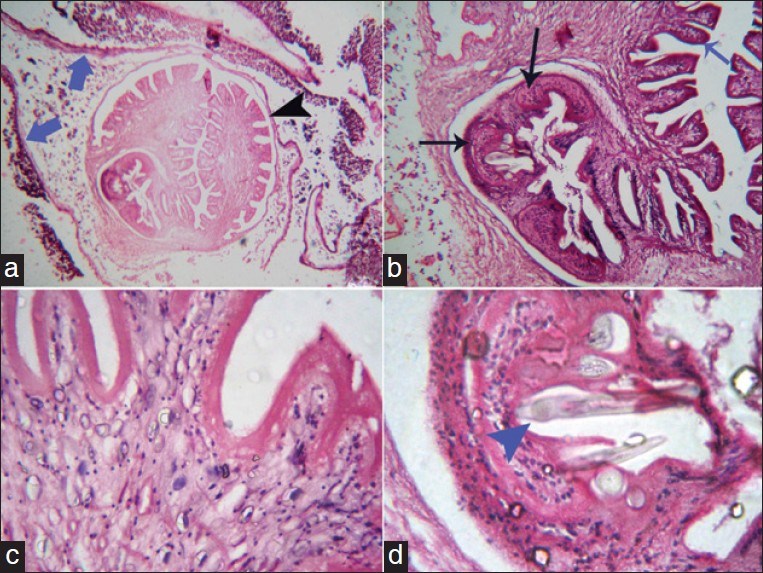 |
Figure 4: (a) Larval taeniasolium cyst in a histopathological section. An entire cysticercus seen within the bladder walls (blue arrows) with scolex (black arrowhead) (b) Higher magnification (100×) of the cyst: The parenchymatous portion with the extensive folding of the spiral canal (blue arrow) and one sucker (black arrows) of the scolex. Calcareous corpuscles can be seen in the fibrous tissues of the sucker. (c). 400×: Parenchymatous portion (d) Hooklet
Click here to view |
|
Table 1: Age distribution of the patients as seen in our study
Click here to view |
|
Table 2: Site distribution of the lesions of the patients as seen in our study
Click here to view |
|
Table 3: Distribution of cases with regard to provisional diagnoses as seen in our study
Click here to view |
Cysticercosis has been termed as a “biological marker” of the social and economic development of a community. [6] It is a major public health problem, especially in the developing world, being endemic in Mexico, Central and South America, Asia, India, sub-Saharan Africa and China. [1],[4],[5],[6],[7] Taenia solium taeniosis and cysticercosis are diseases associated with poverty, pork consumption and poor pig husbandry practices. [8] Humans are the only definitive hosts of T. solium harbouring adult tapeworm in their intestines (taeniasis), where as both man and pig can act as intermediate hosts and harbour the larvae in different internal organs (cysticercosis) including brain. [6],[9],[10],[11] Cysticercosis in both humans and pigs is acquired through ingestion of eggs excreted in faeces by human carrier. [6],[12] Cysticercosis is a disease caused by the encysted larval stage of the tapeworm taenia solium following ingestion of unwashed vegetables or poorly cooked meat contaminated with the eggs of the worm or by auto-infection. [1],[2],[9],[11],[12],[13],[10] When humans ingest eggs, through fecal-oral transmission or possibly through autoinfection, they become dead-end hosts of the larval stage of the parasite and develop cysticercosis similar to pigs. [1],[9] Fecal-oral contamination usually occurs via infected food handlers who do not properly wash their hands before working, or by fruit and vegetables fertilized with contaminated human waste. Autoinfection involves the retrograde transmission of proglottids from the intestines into the stomach with subsequent release of T. solium eggs into the human gut. [1],[9] The eggs hatch in the upper intestine and the embryos migrate via lymphatics or blood to various organs of the body. [2] As blood is the prime transport medium for this parasite, it has a high propensity for localization in those organs which have an ample blood supply, such as, the brain, muscles and subcutaneous tissues. [2],[14],[15],[16],[17] The cestode has high tropism to cholinergic tissues. (neuromuscular junctions rich in acetylcholine esterase) [18] All the swellings which were seen in our study were subcutaneous or intramuscular in nature.
Clinically alone, cysticerci nodules in the skin are difficult to differentiate from benign mesenchymal tumors and lymphadenitis. The cytomorphological identification of larvae in FNAC smears by different workers has improved the diagnostic utility of FNAC in skin nodules and hence prevent a diagnostic and therapeutic error. [3],[15],[19],[20],[21] FNAC is a well recognized diagnostic procedure for evaluation of subcutaneous cysticercosis. [14] The viable cysts yield clear, pearly white fluid and show fragments of bladder wall with tiny parasitic nuclei in a clear acellular background, which were observed in our study. Viable cysticerci may not cause any inflammatory response. [3],[14],[20] Aspiration of clear fluid is a strong pointer towards parasitic infestation, although in a significant number of cases, aspirate may also be purulent or hemorrhagic. [16],[22] Aspirates of necrotic lesions may contain fragments of bladder wall, including calcareous corpuscles and detached single hooklets, which was seen in one case in our study. These hooklets are characterized by the presence of a refractile pointed end and a non-refractile, bifurcated blunt end. [15],[16],[17] However, when the cysticerci degenerate, there is an infiltration of inflammatory cells, associated with the development of foreign body granulomas. [3],[14],[20] Single detached hooklets and calcareous corpuscles may be the only recognizable remnants in aspirates of calcified cysts. [3],[14],[16],[17]
The suspicion of cysticercosis is raised if the smears show the presence of eosinophils, neutrophils, palisading histiocytes or giant cells. A definitive diagnosis of cysticercosis requires the demonstration of fragments of larval cuticle and parenchyma, calcareous corpuscles, hooklets or scolex. [3],[14],[20] Fully developed cysticerci are opalescent, milky white cysts, elongated to oval and about 1 cm in diameter. The cyst contains fluid and a single invaginated scolex. The scolex has a rostellum, four suckers and 22-32 small hooklets. The cyst wall is multilayered, 100-200 mm thick and covered by microvilli. The outer cuticular layer is smooth, hyalinized and is frequently thrown into projections. [3],[14],[16],[19] Beneath the tegument is a row of tegumental cells. The inner layer or parenchyma is loose and reticular, containing mesenchymal cells and calcareous corpuscles. [3],[14],[16],[20] The calcareous corpuscles are a unique feature of cestode tissue. These spherical, non-cellular masses occur in the parenchyma and are especially prominent in larval cestodes. The corpuscles are seen as small, dense basophilic-purple round structures either singly or in clusters in hematoxylin and eosin stain, [15],[16],[23] which were also seen in our study.
Another unusual cause for subcutaneous swelling is the hydatid cyst, and cytomorphological details help to distinguish it from cysticercus. The bladder wall is thin, membranous in cysticercus whereas it is thicker, acellular and lamellated in a hydatid cyst. [16],[17],[23] Cysticercus has only one scolex which is large, almost 1 mm in diameter with two rings of alternating large and small hooklets measuring 170 μm and 130 μm, respectively. The scolex is visible to the unaided eye and along with the hooklets, can be easily recognized at scanning magnification. [16] In contrast, hydatid cysts have multiple daughter cysts within a parent cyst, and hence may yield many scolices in a clear aspirate. In echinococcus, individual scolices are small and the hooklets measure 22 μm and 40 μm, and can be appreciated only at higher magnification. [16],[23] Parasitic subcutaneous and intramuscular nodules are also produced by coenuri, the larvae of tapeworms of the genus Multiceps, and spargana, the larvae of Spirometramansonoides. The stroma and tegment of these two larvae closely look like that those of cysticercus. Cysticerci and coenuri have suckers and hooklets, whereas spargana do not. Most cases by Coenurus are seen in tropical climates and Southern Africa. Spargonosis is seen worldwide, but most commonly in China, Japan and South-east Asia. [20]
The most serious and potentially fatal clinical feature of cysticercosis arises when the organism invades the central nervous system (CNS). The usual symptoms are headache, vomiting, seizure, convulsion, mental deterioration, or impaired visual acuity. [24] Hence, early diagnosis and prompt treatment of subcutaneous cysticercosis would prevent such CNS complications. Any patient presenting with a subcutaneous or intramuscular cysticercus nodule should have a cranial computed tomography (CT) done to rule out neurocysticercosis.FNAC is a quick, reliable and low-cost outpatient procedure for diagnosis of subcutaneous nodules caused by cysticercosis. It is one of the tools for preoperative diagnosis and may even prevent the need for open biopsy and subsequent histopathological examination, as the parasite may not be demonstrated even on biopsy specimens. [3],[15],[16] The cytological diagnosis is quite straightforward in cases where the actual parasite structure is identified in the smears. However, in other cases, the presence of eosinophils, histiocytes and a typical granular dirty background are the features which should always alert the pathologist to this possibility. In some cases of cysticercosis, none of these features may be present, and the inflammatory infiltrate may also be variable. Cysticercosis is more common than previously thought. In all inflammatory/cystic lesions, especially in endemic areas, the possibility of cysticercosis should be kept in mind irrespective of age, location, associated pain with the lesions and the size of the lesion. [3],[15]
Improving one’s personal hygiene, taking appropriate preventive measures and deworming with anti-parasitic medications under medical guidance can help decrease the prevalence of this disease. Prompt diagnosis of subcutaneous cysticercosis with easy procedures like FNAC and simple treatment with albendazole can help eliminate the disease before the dangerous neurocysticercosis develops.
In a developing country like India, cultivating crops and vegetables in water contaminated with the infected eggs (sewage) can transmit cysticerci to humans. In such situations, a rapid, safe and reliable cytologic diagnosis of subcutaneous cysticercosis by FNAC on an outpatient basis proves to be a cost-effective procedure. In endemic areas with subcutaneous swellings, cysticercosis should always be kept on top of the list of differential diagnoses. An early diagnosis and prompt institution of therapy for such subcutaneous lesions is essential to prevent dangerous sequelae. Our results support the utility and importance of FNA cytology in the diagnosis of cysticercosis. A thorough search for the parasite fragment in acute suppurative lesions, especially in endemic areas, is warranted, as an infected parasitic cyst can mimic a suppurative process. The eyes see only when the mind knows what and where to search.
| 1. |
Kraft R. Cysticercosis: An emerging parasitic disease. Am Fam Physician 2007;76:91-6. |
| 2. |
Sodhi PK, Ratan SK. Submandibular lymph node enlargement due to cysticercosis infestation. Scand J Infect Dis 2004;36:227-9. |
| 3. |
Gill M, Dua S, Gill P, Gupta V, Gupta S, Sen R. Cytomorphological spectrum of subcutaneous and intramuscular cysticercosis: A study of 22 cases. J Cytol 2010;27:123-6. |
| 4. |
Naik D, Srinath MG, Kumar A. Soft tissue cysticercosis-Ultrasonographic spectrum of the disease. Indian J Radiol Imaging 2011;21:60-2. |
| 5. |
Kamal MM, Grover SV. Cytomorphology of subcutaneous cysticercosis. A report of 10 cases. ActaCytol 1995;39:809-12. |
| 6. |
Prasad KN, Prasad A, Verma A, Singh AK. Human cysticercosis and Indian scenario: A review. J Biosci 2008;33:571-82. |
| 7. |
Rao RN, Krishnani N, Malhotra K, Suresh B, Mehrotra R. Dilemmas in cytodiagnosis of subcutaneous swellings: Mimics and look-alikes of cysticercosis. J ClinPathol 2010;63:926-9. |
| 8. |
Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taeniasoliumtaeniosis/cysticercosis in Asia: Epidemiology, impact and issues. Acta Trop 2003;87:53-60. |
| 9. |
Adhikari RC, Aryal G, Jha A, Pant AD, Sayami G. Diagnosis of subcutaneous cysticercosis in fine needle aspirates: A study of 10 cases. Nepal Med Coll J 2007;9:234-8. |
| 10. |
Agarwal A, Murty OP, Jain M. Fine needle aspiration cytology in the diagnosis of cysticercosis cases. Asian Pac J Trop Med 2009;2:49-53. |
| 11. |
Kung IT, Lee D, Yu HC. Soft tissue cysticercosis. Diagnosis by fine needle aspiration. Am J ClinPathol 1989;92:834-5. |
| 12. |
Amatya BM, Kimula Y. Cysticercosis in Nepal: A histopathologic study of sixty-two cases. Am J SurgPathol 1999;23:1276-9. |
| 13. |
Lakhey M, Hirachand S, Akhter J, Thapa B. Cysticerci in palpable nodules diagnosed on fine needle aspiration cytology. JNMA J Nepal Med Assoc 2009;48:314-7. |
| 14. |
Batrani M, Kaushal M, Chaturvedi NK, Yadav R. Fine needle aspiration of subcutaneous cysticercosis. DiagnCytopathol 2010;38:347-8. |
| 15. |
Rao RN, Krishnani N, Malhotra K, Suresh B, Mehrotra R. Dilemmas in cytodiagnosis of subcutaneous swellings: Mimics and look-alikes of cysticercosis. J ClinPathol 2010;63:926-9. |
| 16. |
Handa U, Garg S, Mohan H. Fine needle aspiration in the diagnosis of subcutaneous cysticercosis. DiagnCytopathol 2008;36:183-7. |
| 17. |
Arora VK, Gupta K, Singh N, Bhatia A. Cytomorphologic panorama of cysticercosis on fine needle aspiration. A review of 298 cases. ActaCytol 1994;38:377-80. |
| 18. |
Vuong PN. Fine needle aspiration cytology of subcutaneous cysticercosis of the breast. Case report and pathogenic discussion. ActaCytol 1989;33:659-62. |
| 19. |
Kamal MM, Grover SV. Cytomorphology of subcutaneous cysticercosis. A report of 10 cases. ActaCytol 1995;39:809-12. |
| 20. |
Verma K, Kapila K. Fine needle aspiration diagnosis of cysticercosis in soft tissue swellings. ActaCytol 1989;33:663-6. |
| 21. |
R. Patnayak, D. Kalyani, I. SatishRao, A. Prayaga, C. Sundaram, A. Jena: Cysticercosis: The Hidden Parasite With Short Review Of Literature. The Internet Journal of Infectious Diseases. 2007 Volume 6 Number 1. DOI: 10.5580/1ac2. |
| 22. |
Khurana N, Jain S. Cytomorphological spectrum of cysticercosis – A review of 132 cases. Indian J PatholMicrobiol 1999;42:69-71. |
| 23. |
Singh N, Arora VK, Bhatia A. Are all subcutaneous parasitic cysts cysticercosis? ActaCytol 2006;50:114-5. |
| 24. |
McAdam AJ, Sharpe AH. Infectious diseases. In: Kumar V, Abbas AK, Fausto N, Aster, editors. Robbins and Cotran Pathologic Basis of Disease. 8 th ed. Philadelphia: Saunders; 2010. p. 331-99. |
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1755-6783.116522
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
[Table 1], [Table 2], [Table 3] |




