|
The case of a 38-year-old man suffering from hydatid cyst located in the gallbladder is presented. Although Morocco remains an endemic area for echinococcosis, this presentation of the disease is rare. Pericyst was tightly attached to the liver. Complete pericystectomy followed by cholecystectomy was done. Histopathology confirmed the presence of calcified hydatid cyst of the gallbladder. Perioperative adjuvant medical therapy with albendazole was administered. In a 2-year follow-up, no recurrence has occurred.
Keywords: Echinococcal cyst, gallbladder, hydatid disease, porcelain gallbladder
How to cite this article:
Rabbani K, Narjis Y, Louzi A, Benelkhaiat R, Jalal H, Finech B. Unusual localization of hydatidosis: Hydatid cyst of gallbladder. Ann Trop Med Public Health 2011;4:119-21 |
How to cite this URL:
Rabbani K, Narjis Y, Louzi A, Benelkhaiat R, Jalal H, Finech B. Unusual localization of hydatidosis: Hydatid cyst of gallbladder. Ann Trop Med Public Health [serial online] 2011 [cited 2017 Nov 14];4:119-21. Available from: https://www.atmph.org/text.asp?2011/4/2/119/85766 |
Hydatid disease is an endemic zoonosis caused by larval stages of dog tapeworms belonging to the genus Echinococcus. The most common affected organs are the liver (75%) and the lungs (15%). [1],[2] This disease continues to be a substantial cause of morbidity and mortality in many parts of the world, such as the countries bordering the Mediterranean Sea, the Middle East, the South America, the South Africa and Oceania. [3] Echinococcal cyst located in the gallbladder constitutes an extremely rare manifestation of the hydatid disease. In the literature, only a few cases concerning involvement of the gallbladder in hydatid disease are reported. This paper emphasizes that echinococcal cyst should be included in the differential diagnosis in cases of cystic masses of the gallbladder, especially in endemic areas like Morocco.
A 38-year-old Moroccan gentleman, resident of a sheep-rearing area, previously in good health, was admitted to our hospital with fever. His illness had started 6 days earlier with fever followed by abdominal pain, nausea and vomiting. Other medical history was unremarkable. On examination, he looked ill but was conscious, oriented and febrile (38°C). Abdominal examination revealed right upper quadrant tenderness, with Murphy’s sign positive and a palpably enlarged liver 3 cm below the right costal margin. Rest of his examination was unremarkable. Laboratory findings on admission showed a white blood cell count of 12,800 mm 3 (62% neutrophils, 14% eosinophils); erythrocyte sedimentation rate, 23 mm in the first hour. Alanine-aminotransferase (ALT), aspartate-aminotransferase (AST) and total bilirubin were normal. The diagnosis, supported by ultrasound, was an hydatid cyst of the gallbladder [Figure 1]. Chest x-ray showed no signs of localization in the lung.
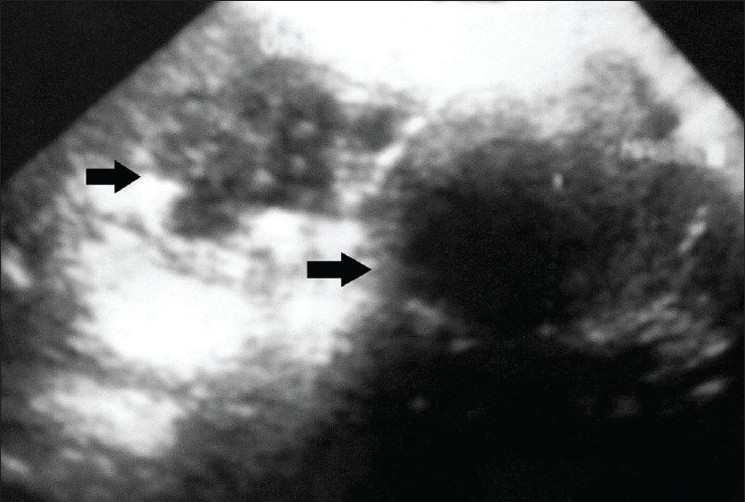 |
Figure 1: An infected hydatid cyst partially calcifi ed of the liver with involvement and deformation of the gallbladder in ultrasound
Click here to view |
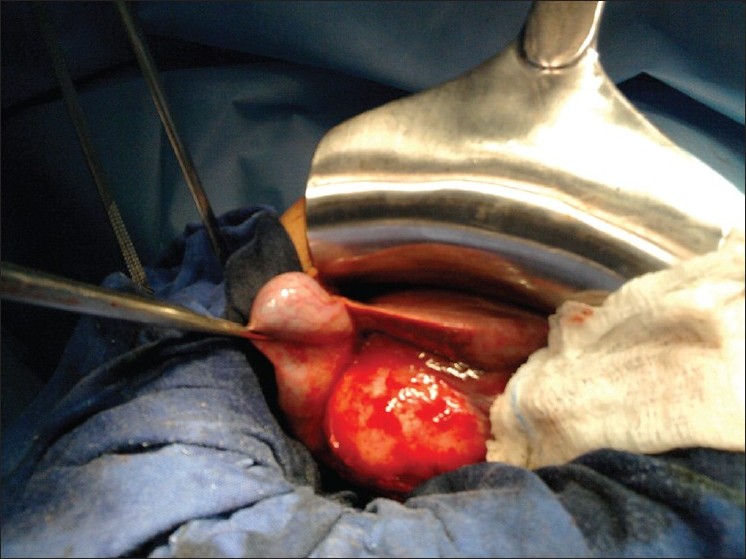 |
Figure 2: Operative view: Calcifi ed hydatid cyst (dimensions, 5 cm × 3 cm) located in the body of the gallbladder
Click here to view |
In accordance with the assumed diagnosis of infected hydatid cyst of the gallbladder, antibiotics were administered; the patient became afebrile after 36 hours. The pain subsided and hepatic tenderness gradually diminished. After about 1 week of improvement, there was recurrence of the fever and abdominal pain. A laparotomy performed in the subsequent week showed thickening of the gallbladder wall, with a calcified hydatid cyst (dimensions, 5 cm × 3 cm) located in the body of the gallbladder [Figure 2]. Complete pericystectomy along with cholecystectomy was performed; the abdomen was then carefully packed with pads soaked in hypertonic saline solution to avoid spillage of the echinococcal fluid. No other cysts were found during careful exploration of the liver. Histopathology of the surgical specimen confirmed the diagnosis of a gallbladder hydatid cyst. The patient’s immediate postoperative recovery was uneventful, and he was discharged on the 8 th postoperative day. The patient received tree 21-day courses of oral Albendazol 400 mg/day with 10 days pause in between. Two years of clinical and ultrasonographic follow-ups have shown no recurrence.{Figure 2}
Hydatid disease is a parasitic tapeworm disease caused by the larval stage of Echinococcus granulosus or Echinococcus multilocularis. The main species pathogenic for humans in Mediterranean and Southern European countries is Echinococcus granulosus. [3] The liver is the most frequently involved organ in both forms, followed by the lung. [1],[2] Excluding liver and lungs, all the other organs of the human body are considered as uncommon sites of localization of the hydatid disease. In 10% of all cases of echinococcosis, the localization of the cyst is characterized as rare and involves the spleen, pancreas, gallbladder, adrenal gland, pelvis, seminal vesicle, heart, bone, breast, kidney, thyroid gland and soft tissues.
Pathogenesis of the primary gallbladder hydatid cysts (GHCs) is divided depending on the location of the cyst. In the lumen of the gallbladder or on the external surface, [4],[5] it is usually the result of either intrabiliary rupture of a hepatic hydatid cyst or of a direct cyst rupture into the gallbladder. [6] A primary hydatid cyst of the gallbladder is even more uncommon, with only a few cases reported in the literature. [7],[8] Rigas et al. described this localization to be a result of brood capsules dissemination through the lymphatic rather than biliary spread in primary gallbladder hydatid disease; unlike Cangiotti et al., who found the dissemination to occur through the biliary tract.
Diagnosis of hydatid disease with an uncommon primary location may be a diagnostic problem even in regions where the disease is endemic. [9] Most patients suffering from echinococcosis are asymptomatic, especially in the lengthy early stages. Symptoms appear because the size of the cyst, usually bigger than 5 cm in diameter, causes pressure-related problems or because rupture of the cyst produces infection in the affected organ or allergic reaction. In some cases, the allergic reaction can lead to anaphylactic shock. Apart from history of close contact with dogs; or the presence of pain, mid-abdominal discomfort and dyspepsia, or swelling mass on physical examination, the diagnosis of hydatid cyst relies on serological tests and imaging techniques. Ultrasound and computed tomography are very helpful in the diagnosis of hydatid cysts; they may detect hydatid disease in the form of purely cystic lesions, or when floating membranes, daughter cysts, or vesicles are recognized. [10] Ultrasound features of GHCs are similar to those of hepatic hydatid cysts. [11] The classification proposed by Gharbi [12] can be adopted for other locations. Type I appears cystic and unilocular. Type II is a fluid filled with a floating membrane (the water lily sign). Type III has a typical honeycomb appearance. Type IV is a heterogeneous mass, and type V is a calcified lesion. However, although types II and III are specific to GHCs, there is no pathognomonic image for types IV and V. In our case, the initial ultrasound described the lesion as an acute cholecystitis with infected hydatid cyst partially calcified of the liver with involvement and deformation of the gallbladder. Gallbladder hydatid cysts should be differentiated from a gallbladder carcinoma in types IV and V and other extra-hepatic cystic lesions. [4]
Surgical intervention is the optimal treatment for GHCs; in the present case, total pericystectomy with cholecystectomy was performed. [13] Some authors recommend using a scolicidal agent in the operating field to avoid dissemination in case of rupture. [14] Mebendazole or albendazole should be used as an adjunct to surgery when the resection is incomplete, or as a treatment when surgery cannot be done. [13],[14]
| 1. |
Safioleas M, Misiakos E, Manti C, Katsikas D, Skalkeas G. Diagnostic evaluation and surgical management of hydatid disease of the liver. World J Surg 1994;18:859-65. |
| 2. |
Safioleas MC, Misiakos EP, Kouvaraki M, Stamatakos MK, Manti CP, Felekouras ES. Hydatid disease of the liver: A continuing surgical problem. Arch Surg 2006;141:1101-8. |
| 3. |
Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis 2007;7:385-94. |
| 4. |
Safioleas M, Stamoulis I, Theoqaris S, Moulakakis K, Makris S, Kostakis A. Primary hydatid disease of the gallbladder: A rare clinical entity. J Hepatopancreat Pancreat Surg 2004;11:352-6. |
| 5. |
Cangioti L, Muiesan P, Begni A, De Cesare V, Pouche A, Giulini S, et al. Unusual localizations of hydatid disease; an 18 years experience. G Chir 1994;15:83-6. |
| 6. |
Kapoor A, Sarma D, Gandhi D. Sonographic diagnosis of a ruptured primary hydatid cyst of the gallbladder. J Clin Ultrasound 2000;28:51-2. |
| 7. |
Rigas AM, Karatzas GM, Markidis NC, Bonikos DS, Sotiropoulou GG, Skalkeas G. Primary hydatid cyst of the gallbladder. Br J Surg 1979;66:406. |
| 8. |
Raza MH, Harris SH, Khan R. Hydatid cyst of the gallbladder. Indian J Gastroenterol 2003;22:67-8. |
| 9. |
Kiresi DA, Karabacakoglu A, Odev K, Karakose S. Uncommon locations of hydatid cysts. Acta Radiol 2003;44:622-36. |
| 10. |
Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics 2003;23:475-94. |
| 11. |
Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examinatoin of hydatic liver. Radiology 1981;139:459-63. |
| 12. |
Gharbi HA, Hassine W, Abdesselem K. Ultrasonography in abdominal hydatid disease. Comments and specific aspects (Echinococcus granulosus) Ann Radiol 1985;28:3134. |
| 13. |
Wani RA, Malik AA, Chowdri NA, Wani KA, Naqash SH. Primary extrahepatic abdominal hydatidosis. Int J of Surgery 2005;3:125-7. |
| 14. |
Ivanis N, Rubinic M, Gudovic A, Zeider F. Ultrasound image of an echinococcus daughter cyst in the gallbladder. Ultraschall Med 1994;15:269-71. |
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1755-6783.85766
[Figure 1], [Figure 2] |





