| Abstract |
Background and Objective: For many years, extended-spectrum-beta-lactamase (ESBL)-producing bacteria were problems mainly located in medical facilities. Within the last decade, however, ESBL-producing bacteria have started spreading into the community and the hospital. The aims of this study were to detect ESBL-producing uropathogenic Escherichia coli (UPEC), susceptibility to antibiotics, and β-lactamase genes. Materials and Methods: Two hundred bacterial isolates were collected from inpatients and outpatients in the west of Iran from December 2011 to February 2013. All isolates were identified by conventional bacteriological tests. Antimicrobial susceptibility and interpretation were assessed by disk diffusion. ESBL-producing E. coli isolates were tested by polymerase chain reaction (PCR) assay for blaTEM , blaSHV , blaCTX-M , blaOXA-I , and blaOXA-II genes. Results: Of 200 UPEC, 22% (n = 44) were ESBL-producing; 0% showed the least resistance to imipenem, and 97.7% showed high resistance to carbenicillin and ampicillin. The ESBLs CTX-M, SHV, TEM, OXAI, and OXAII were detected in 93.3%, 68.2%, 43.2%, 31.8%, and 22.7% of isolates respectively. Conclusion: A prevalence of ESBL-producing UPEC was observed in outpatients and inpatients; the high levels of resistance to different antibiotics show that treatment options are limited and that infection control measures remain of high importance.
Keywords: CTX-M, extended-spectrum beta-lactamase (ESBL), OXAI, OXAII, PCR, SHV, TEM, uropathogenic Escherichia coli (UPEC)
| How to cite this article: Mohajeri P, Rostami Z, Farahani A, Norozi B. Distribution of ESBL producing Uropathogenic Escherichia coli and carriage of selected β-lactamase genes in Hospital and community isolates in west of Iran. Ann Trop Med Public Health 2014;7:219-22 |
| How to cite this URL: Mohajeri P, Rostami Z, Farahani A, Norozi B. Distribution of ESBL producing Uropathogenic Escherichia coli and carriage of selected β-lactamase genes in Hospital and community isolates in west of Iran. Ann Trop Med Public Health [serial online] 2014 [cited 2021 Jan 23];7:219-22. Available from: https://www.atmph.org/text.asp?2014/7/5/219/154823 |
| Introduction |
Urinary tract infections (UTIs) may be an emerging problem in patients in various parts of the world. [1] Cephalosporins are commonly used to treat infections due to Escherichia coli in UTIs. [2] However, resistance to these antibiotics is on the rise all over the world, and the increase of antibiotic resistance is a crisis for the community and for hospitals. [3]
This phenomenon has been attributed to the emergence of strains that can produce extended-spectrum beta-lactamase (ESBLs). [4] The strains can produce plasmid-mediated enzymes capable of hydrolyzing cephalosporins and monobactams, and represent major challenges and serious consequences for antibiotic therapy. [5]
The defined ESBL variants have been growing in number, and more than 100 different ESBL variants are known at present. [6]
TEM, SHV, and CTX-M ESBLs have been the dominant ESBL gene families in the world in recent years. These genes are genetically diverse and highly mobile. [7] These plasmid-located genes lead to the dynamic evolution and epidemiology of ESBLs. [8] ESBLs have compromised cause the crisis of antimicrobial drug resistance. [9] These genes can be isolated from both inpatients and outpatients. [10]
UTIs are the most common type of community-associated ESBL infections caused by E. coli. Therefore, ESBL-producing E. coli from UTIs constitute a feasible bacterial population for a comparative study. [11]
The aim of this study was to detect ESBL-producing uropathogenic E. coli (UPEC) and evaluate their in vitro susceptibility to antibiotics, particularly to cephalosporins; finally, isolates were tested for the presence of five different beta-lactamase gene families.
| Materials and Methods |
Bacterial isolates
UPEC were collected from the hospitals and the community in the west of Iran from December 2011 to February 2013. All isolates were identified by conventional bacteriological tests. A hospital infection was defined as an infection that occurred >48 h after hospital admission, and the community samples were isolated from patients who had no history of hospitalization. [12]
Susceptibility testing and ESBL detection
Antimicrobial susceptibility and interpretation were tested by disk diffusion according to the Clinical and Laboratory Standards Institute standards. [12] The following antibiotics were used: ceftazidime (CAZ: 30 μg), ceftriaxone (CRO: 30 μg), cefotaxime (CTX: 30 μg), piperacillin (PIP: 100 μg), gentamicin (GM: 10 μg), amikacin (AN: 30 μg), imipenem (IMP: 10 μg), ciprofloxacin (CIP: 5 μg), ampicillin (AM: 10 mg), azithromycin (AZ: 10 mg), and trimethoprim/sulfamethoxazole (SXT: 30 μg) (MAST Group Ltd., Liverpool, UK). Disks containing 30 μg each of ceftazidime, ceftriaxone, cefotaxime, and cefepime, plus 10 μg of clavulanic acid were placed on Mόller-Hinton agar for detection of ESBL-producing bacteria. A positive test result was defined as a ≥5-mm increase in inhibition zone diameter, compared to a disk without clavulanic acid. [13]
PCR amplification
Screening of ESBL-producing UPEC was performed using PCR assay for blaTEM , blaSHV , blaCTX-M , blaOXA-I , and blaOXA-II genes using the primers described in [Table 1].
| Table 1: Primers of polymerase chain reaction (PCR)
Click here to view |
Standard PCR protocols and conditions for CTX-M were modified in the following way.
The amplified products were separated in 1% agarose gel. The gel was visualized by staining with ethidium bromide. A 100 bp ladder molecular weight marker (Roche, Life Science, Germany) was used to measure the molecular weights. The images were digitized using a gel documentation system.
Statistical analysis
A statistical comparison of the frequencies of ESBL presence in the UPEC isolates was made using the chi-square test. A P value <0.05 was considered significant.
| Results |
Out of 200 samples, 22% (n = 44) were shown to produce ESBLs by the disk diffusion test, or 68.18% of the samples isolated from the community and 31.82% of samples from the hospital. Our study showed the rates of resistance ranged from the least resistance, to imipenem, at 0%); to high resistance to carbenicillin, ampicillin, piperacillin, and cefuroxime, at >90%; and to imipenem and amikacin, at <10% [Table 2].
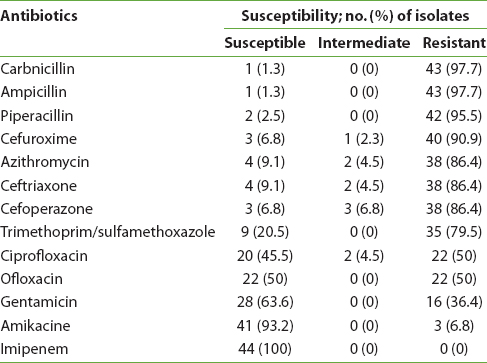 |
Table 2: Antimicrobial-susceptibility for ESBL-producing uropathogenic Escherichia coli (UPEC) isolates
Click here to view |
Molecular analysis was done by amplification of DNA in 44 ESBL isolates with specific primers for blaTEM, blaSHV, and blaCTX-M-1 genes [Figure 1]. CTX-M ESBLs were detected in 93.3% (n = 41) ESBL producers, while TEM, SHV, OXAI, and OXAII ESBLs were found in 68.2% (n = 30), 43.2% (n = 19), 31.8% (n = 14), and 22.7% (n = 10), respectively.
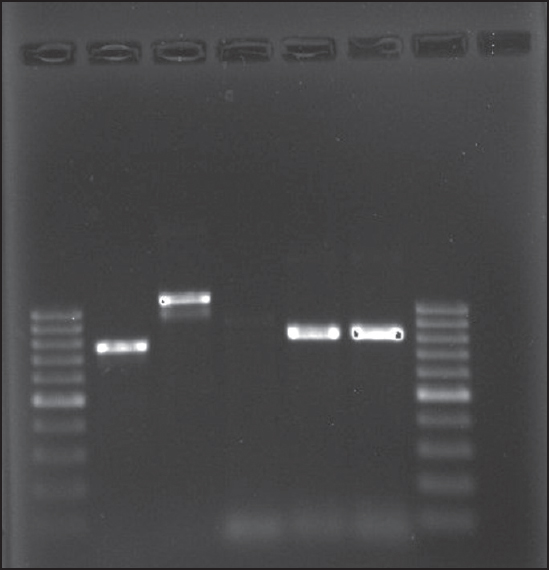 |
Figure 1: Lane 1&7: Ladder 100 bp; lane 2: CTX-M; lane 3: TEM; lane 4: SHV; lane 5: OXAII; lane 6: OXAI
Click here to view |
A significant association between ESBL-producing UPEC and CTX-M was observed with respect to resistance to different cephalosporins, such as cefuroxime [P < 0.0001], and other antibiotics such as ampicillin [P < 0.0001], carbenicillin [P < 0.0001], and trimethoprim/sulfamethoxazole [P < 0.040] [Table 3].
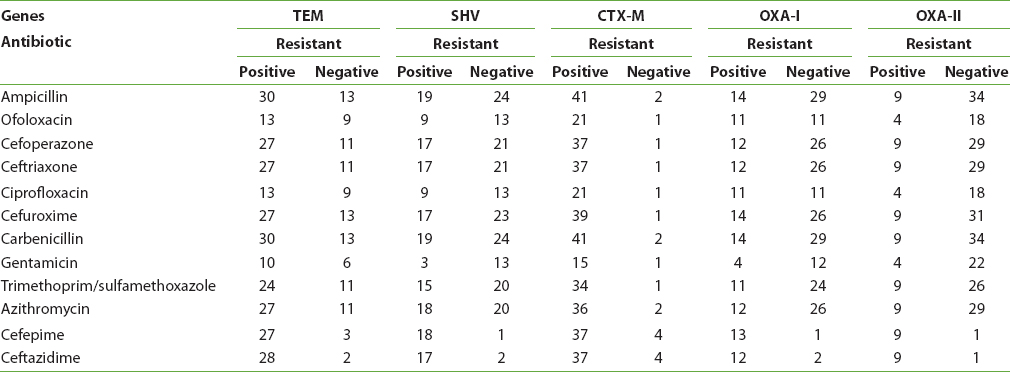 |
Table 3: Antimicrobial susceptibility for each gene
Click here to view |
SHV-positive isolates were significantly resistant to gentamicin [P < 0.013].
OXAI-positive isolates were resistant to ofoloxacin and ciprofloxacin [P < 0.010], [P < 0.0001].
Thirty-four UPEC isolates carrying CTX-M, 25 isolates with TEM, and 16 isolates with SHV were resistant to cefepime, ceftazidime, ceftriaxone, and cefuroxime [Table 3].
| Discussion |
UTIs are regarded as a health problem around the world and are common in both inpatients and outpatients. [14] UTIs caused by ESBL-producing organisms are a cause of concern due to the decreasing clinical treatment protocols. The results of our study show that significant resistance was presented to carbenicillin, ampicillin, azithromycin, piperacillin, cefuroxime, ceftriaxone, trimethoprim/sulfamethoxazole, ciprofloxacin, and ofloxaxin by the UPEC isolates. This adds up to an alarming resistance to these antibiotics. Regarding TEM, SHV, CTX-M, OXAI, and OXAII enzymes, the findings of this study show the spread of these enzymes in the inpatient and outpatient isolates in the west of Iran. However, the fact of CTX-M-TEM and SHV production is of concern, especially that in E. coli, because these enzymes are commonly encoded by plasmids. E. coli are important because it has been reported that isolates carrying ESBL genes had a greater resistance to various classes of antibiotics and these genes are located on plasmids, transposons, or integrons. [15] The current distribution of ESBL in patients with community-acquired UTIs is a generalized problem, thus producing a flow of isolates from the inpatients and outpatients. [16] According to the results, 68.18% of the samples were isolated from patients who had no history of hospitalization, According to these statistics we propose, there are two main reasons for this development. First, many patients self-medicate; and second, there is the widespread use of antibiotics and access to uncontrolled drugs (taken without any antibiotic susceptibility testing and lack of attention to laboratory screening). Furthermore, this study showed high levels of resistance to antibiotics, and it also showed that horizontal gene transfer plays an important role in spreading resistance to many different strains, species, and into different reservoirs.
This finding suggests that gene coding for ESBLs and gene coding for resistance to these antibiotics may reside within the same plasmids and, therefore, be spread together.
Briefly, the prevalence of ESBL E. coli was observed in our hospitals and in the community; the high levels of resistance to different antibiotics show that treatment options are limited and infection control measures remain of high importance.
| Acknowledgment |
This work was performed in partial fulfillment of the requirements for M.Sc thesis in Microbiology (Mrs. Baharak Norozi).The authors would like to acknowledge Kermanshah University of Medical Sciences. The study was financially supported by the Kermanshah University of Medical Sciences for grant 91119.
| References |
| 1. |
Cormican M, Morris D, Corrbet-Feeney G, Flynn J. Extended spectrum beta-lactamase production and fluorquinolone resistance in pathogens associated with community acquired urinary tract infection. Diagn Microbiol Infect Dis 1998;32:317-9.
|
| 2. |
Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect 2007;55:254-9.
|
| 3. |
Reinthaler FF, Feierl G, Galler H, Haas D, Leitner E, Mascher F, et al. ESBL-producing E. coli in Austrian sewage sludge. Water Res 2010;44:1981-5.
|
| 4. |
Scoulica EV, Neonakis IK, Gikas AI, Tselentis YJ. Spread of bla (VIM-1)-producing E. coli in a university hospital in Greece. Genetic analysis of the integron carrying the bla(VIM-1) metallo-beta-lactamase gene. Diagn Microbiol Infect Dis 2004;48:167-72.
|
| 5. |
Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect 2009;73:345-54.
|
| 6. |
Available from: http://www.lahey.org/studies/webt.htm. Available from: http://www.lahey.org/studies. [Last accessed on: 2015 March 27].
|
| 7. |
Zarfel G, Galler H, Feierl G, Haas D, Kittinger C, Leitner E, et al. Comparison of extended-spectrum-β-lactamase (ESBL) carrying Escherichia coli from sewage sludge and human urinary tract infection. Environ Pollut 2013;173:192-9.
|
| 8. |
Jungmin K, Lim YM, Rheem I, Yeonhee L, Je-Chul L, Sung YS, et al. CTX-M and SHV-12 b-lactamases are the most common extended-spectrum enzymes in clinical isolates of Escherichia coli and Klebsiella pneumoniae collected from 3 university hospitalswithin Korea. FEMS 2005;245:93-8.
|
| 9. |
Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: The worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents 2010; 35:316-21.
|
| 10. |
Kuroda H, Yano H, Hirakata Y, Arai K, Endo S, Kanamori H, et al. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli in Japan: Emergence of CTX-M-15-producing E. coli ST131. Diagn Microbiol Infect Dis 2012;74:201-3.
|
| 11. |
Schink AK, Kadlec K, Schwarz S. Analysis of bla(CTX-M)-carrying plasmids from Escherichia coli isolates collected in the BfT-GermVet study. Appl Environ Microbiol 2011;77:7142-6.
|
| 12. |
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care–associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002;137:791-7.
|
| 13. |
Schwaber MJ, Raney PM, Rasheed JK, Biddle JW, Williams P, McGowan JE Jr, et al. Utility of NCCLS guidelines for identifying extended-spectrum beta-lactamases in non-Escherichia coli and non-Klebsiella spp. of Enterobacteriaceae. J Clin Microbiol 2004;42:294-8.
|
| 14. |
Davoodabadi A, Farahani A, Ranjbaran M. Antibiotic resistance in Escherichia coli strains isolated from urine of inpatients and outpatients. Zahedan J Res Med Sci 2012;14:95.
|
| 15. |
Ramazanzadeh R, Farhadifar F, Mansouri M. Etiology and antibiotic resistance patterns of community-acquired extended-spectrum beta-lactamase-producing gram negative isolates in sanandaj. Res J Med Sci 2010;4:243-7.
|
| 16. |
Motta RN, Oliveira MM, Magalhães PS, Dias AM, Aragão LP, Forti AC, et al. Plasmid-mediated extended-spectrum beta-lactamase-producing strains of Enterobacteriaceae isolated from diabetes foot infections in a Brazilian diabetic center. Braz J Infect Dis 2003;7:129-34.
|
Source of Support: None, Conflict of Interest: None
| Check |
DOI: 10.4103/1755-6783.154823
| Figures |
[Figure 1]
| Tables |
[Table 1], [Table 2], [Table 3]



