Context : Varied microbial etiology of parapneumonic effusions/lower respiratory infections casts a heavy burden on rural population in India. Aims : To determine microbial etiology, and burden of parapneumonic effusions (PPE) and lower respiratory infections (LRI), affecting adults hailing from rural areas. Settings and Design : Hospital-based, longitudinal and prospective study during the period of March 2009 to March 2010. Materials and Methods : Sputum, endotracheal (ET) tube secretion, bronchial alveolar lavage (BAL), and pleural fluids were collected from adult patients in intensive care unit (ICU) and wards. Economic burden of hospital stay, treatment, and loss of income for patients/family were noted and analyzed. Keywords: Lower respiratory infections, paraneumonic effusions, pneumonia, rural burden, Stenotrophomonas
The rubric of lower respiratory infections (LRI) can be applied to pneumonia and other types of infections including bronchitis, lung abscess, and emphysema. The greatest potential benefit of microbiological investigation lies in the etiological diagnosis of pneumonia/LRI. [1] The challenge lies not in the clinical diagnosis of pneumonia, but in the laboratory identification of microbial cause. [2],[3] An expanded variety of emerging pathogens provide challenges for the microbiology laboratory. In India, precise studies are required to estimate respiratory health related disease burden in rural areas to plan proper area-specific public health intervention. Reliable data on morbidity and mortality due to LRI’s are scarce in general.
A hospital-based, longitudinal and prospective study was conducted to isolate, identify, and know the prevalence of various microbial pathogens in clinically diagnosed cases of LRI in indoor patients, during the period of March 2009 to March 2010. Sputum, endotracheal secretions, bronchial alveolar lavage (BAL), pleural fluid etc., were the specimens collected. A sputum sample was judged macroscopically valid when the appearance was purulent. It was graded as per Murray and Washington grading system for assessing the quality of the samples. Only grade 5 (epithelial cell/ low power field < 10, and leukocytes/low power field 25) samples were selected for further study. [4] Adult patients of both the sexes were selected for the study. The pathogens were identified by various staining techniques and culture methods, and subjected to antibiotic susceptibility testing, according to Clinical Laboratory Standards Institute (CLSI) recommendations. [5] An attempt has been made to analyze the burden of parapneumonic effusions (PPEs) and LRIs in patients and their families with rural background by taking into consideration the duration of hospital/ICU stay, use of expensive parenteral antibiotics, and loss of work hours etc. Analyses were done using percentages and proportions.
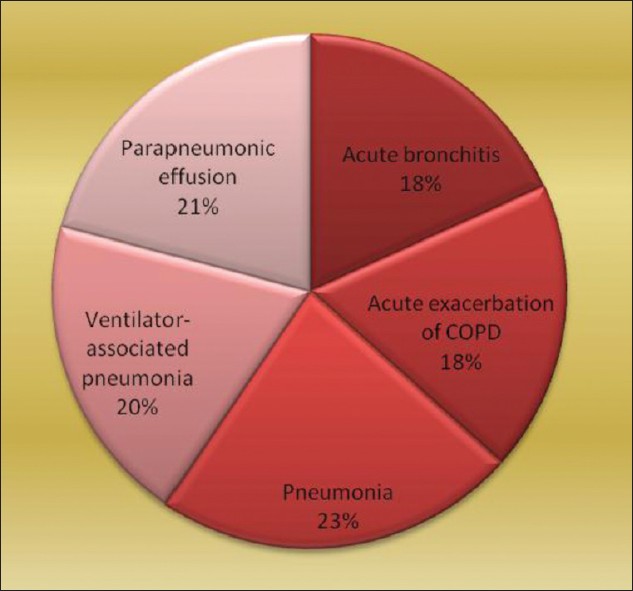
Pie diagram shows relative frequency of different disease types in LRIs. The types and number of specimens collected were sputum (76), endotracheal tube suction (30), BAL (15), and pleural fluid (32) [Figure 1]. Fifty nine out of 76 sputum samples satisfied the selection criteria and were processed further. Thus, total number of specimens processed was 136. Total number of pleural fluid samples was 32.
[Table 1] shows different bacteria isolated from 32 samples of pleural fluid, their percentage, and antibiogram. [Table 2] shows frequency distribution and antibiotic susceptibility pattern of different bacteria isolated from specimens other than pleural fluid.
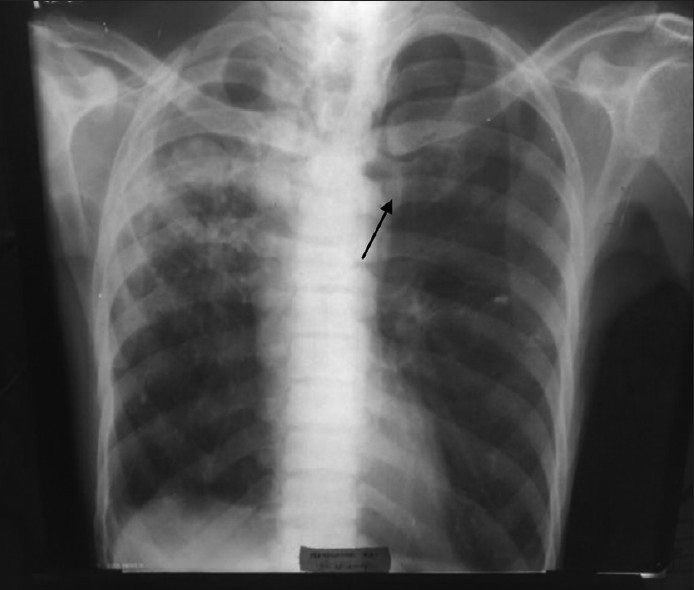
Of the 136 samples processed, bacteria isolated were 41; M. tuberculosis (7) and fungi (11). In 56.62% of specimens, the culture remained sterile after incubation. This observation may be attributed to viral etiology or presence of atypical organisms which cannot be cultured on standard media. Many previous studies also failed to establish a specific etiology in more than half of the cases. [6] Our study established that S. pneumoniae is being recognized as the most important causal bacterium, followed by K. pneumoniae, S. aureus etc. Moraxella catarrhalis was 8.33% in our study, and was slightly higher than that made by Tamang et al (6.90%). [7] We reported 6.25% P. aeruginosa from chronic obstructive pulmonary disease (COPD) patients who had a previous history of hospitalization in the recent past. Three organisms isolated from critically ill, ventilation associated pneumonia cases could not be identified by us by the routine processing. These samples were taken to Calcutta Medical Research Institute (CMRI), Kolkata, for appropriate identification and antibiotic susceptibility testing. They were processed by fully automated VITEK-2 system. Two isolates were identified as “Acinetobacter baumannii”, and one as “Stenotrophomonas maltophilia”. Villers et al. isolated A. baumannii in a quite high number of cases (26.7%) in comparison to our study (4.17%). [8] In their study, infections were favored by the selection pressure of intravenous fluoroquinolones. Intravenous fluoroquinolones were administered very occasionally in our set-up. S. maltophilia was detected only in one case, in our study (2.08%). This observation tallied accurately by the study conducted by Nseir et al. [9] They established COPD and duration of hospital stay were independent risk factors for developing infection by S. maltophilia. The patient from whom it was detected, in our study, was suffering from COPD and required intensive care and ventilator support. Fifteen BAL samples were collected from 15 HIV-infected individuals and stained by Giemsa stain, in addition to the routine processing. Though we failed to identify any Pneumocystis jirovecii, Mycobacterium tuberculosis was identified by Ziehl Neelsen staining in seven samples (14.58%). The isolated S. pneumoniae strains were sensitive to the routine antibiotics except cotrimoxazole. This observation was at par with that made by Hamburger et al.[10] Pneumococci showed good sensitivity to azithromycin, levofloxacin, cefixime, ceftriaxone, and ceftazidime. K. pneumoniae strains were sensitive to ceftriaxone, ceftazidime, and amikacin. S. aureus showed sensitivity to azithromycin, levofloxacin, ceftriaxone, ceftazidime, and amikacin, but not towards cefixime and cotrimoxazole. P. aeruginosa strains were uniformly sensitive to the third generation cephalosporins (ceftriaxone and ceftazidime) and amikacin, and intermediately sensitive to levofloxacin. Antibiotic stewardship practised in the hospital and poor affordability of the patients for purchasing expensive antibiotics may play some role in keeping the organisms sensitive to regular antibiotics. M. catarrhalis showed sensitivity to levofloxacin, cefixime, ceftriaxone, and ceftazidime. According to the study by Tamang et al., ceftriaxone was effective in treating M. catarrhalis infections. [7] Antibiotic susceptibility testing for A. baumannii and S. maltophilia was done by VITEK-2 system, and A. baumannii was found to be multidrug resistant and susceptible only to colistin, ampicillin-sulbactum, cefoperazone-sulbactum, and S. maltophilia to ticarcillin-clavulanate, ceftazidime, and levofloxacin. Candida albicans from nine samples, and Aspergillus fumigatus from two samples, were fungal isolates. Among many cases of Candida isolates, four were from HIV infected individuals with low CD4 counts, and five were from other immunocompromised patients (diabetic patients [2], critically ill in ICU [2], and one on chronic steroid therapy). These nine cases were included in this study, keeping the immune status of the patients in mind, and rest were ignored as colonisers. Aspergillus fumigatus was isolated from two patients. One of the patients was grossly immunosuppressed with fungal ball being visible in X-ray [Figure 2].
Stenotrophomonas maltophilia (previously, Pseudomonas maltophilia, Xanthomonas maltophilia) is the third most common pathogenic, non-fermenting Gram-negative bacilli (NFGNB) worldwide, after Pseudomonas aeruginosa and Acinetobacter calcoaceticus-baumannii complex. [4],[11] The Burkholderia cepacia complex (BCC) and Stenotrophomonas maltophilia are a closely related group of NFGNBs found in many niches of both natural and clinical environments. Both have a similar spectrum of infections ranging from superficial to deep-seated and disseminated infections. [12] BCC and S. maltophilia are exclusive members of the medically important lysine decarboxylase-positive NFGNB group. [4] Stenotrophomonas maltophilia is highly resistant to antibiotics. It causes infections that result in increased morbidity, but not usually mortality, in patients with weakened host defences. The increase in S. maltophilia nosocomial infections is due to the changing nature of the hospital patient population and changes in antibiotic usage. Detection, identification, and susceptibility testing methods require improvement, and this complicates the comparison of published data. Susceptibility testing should be reserved for those isolates that are clearly associated with disease. Treatment can be difficult and may be complicated by biofilm formation. S. maltophilia can both acquire and transfer resistance to antibiotics. [13] Parapneumonic effusions – 32 pleural fluids were processed from patients having parapneumonic effusion. Parapneumonic effusions were associated with bacterial pneumonia, lung abscess, or bronchiectasis. The possibility of a parapneumonic effusion was considered whenever a patient with a bacterial pneumonia was initially evaluated. The presence of free pleural fluid was demonstrated with a lateral decubitus radiograph, computed tomography (CT) of the chest, or ultrasound. Whenever the free fluid separated the lung from the chest wall by >10 mm, a therapeutic thoracentesis was performed. [6] S. pneumoniae was the most frequently isolated organism (26.32%), followed by S. aureus (21.05%) and E.coli (15.79%). In other studies also, Gram-positive organisms were the most common causative agents. [14],[15],[16] Among Gram-negative organisms, E. coli was the commonest. In the study done by Porcel et al., percentages of S. pneumoniae and E. coli were 20.24 and 8.33, respectively. [17] Present study concentrated solely on parapneumonic effusion, where etiology was always bacterial. The study by Porcel et al. took other pleural effusion cases (like malignancy) also into consideration. This difference in the selection of study group may explain the higher occurrence of pneumococci in our study. Over all, S. pneumoniae was the most common organism recovered. This finding may be due to the fact that the primary cause of pleural cavity infection in the series was pneumonia. We found 13 samples to be sterile. When we moved from the initial pathophysiologic stage of a parapneumonic effusion (typical or non-complicated) to later stages (complicated and empyema), pleural fluid yielded positive bacterial cultures more frequently. In our study, Gram-positive bacterial infection of the pleural space was associated with the higher incidence of underlying diseases like HIV-infection and COPD, as similarly shown by the works of Bhattacharya. There S. pneumoniae accounted for 50% of those parapneumonic effusions in AIDS patients. [18],[19],[20] P. aeruginosa was isolated from two patients (10.53%) with thoracostomy tube in-situ. M. tuberculosis was detected in three specimens (15.79%). PPEs and their burden on rural population PPEs are a consequence of bacterial pneumonia. Appropriate, complete treatment of pneumonia can prevent the same which is not being received by the rural population for various reasons (either personal or incorrect treatment modalities practised in villages). The morbidity, mortality, and economic burden due to PPEs in our 32 cases, when analyzed, revealed the following: 1. A total of 20 male and 12 female were adults of 40-60 years age group, hailing from rural areas. 2. The mean number of days of stay in the hospital away from their homes was approximately 10 days, out of which, on an average, three days were in the intensive care unit. 3. Non-invasive (X-ray) and invasive surgical interventions like diagnostic and therapeutic thoracentesis were done on all the cases with a financial burden and risk of nosocomial infections. 4. Parenteral (intravenous) antibiotics and other medicines were life-saving, but expensive. 5. Three patients expired (9.38%), 10 left the hospital against medical advice, and the rest recovered. 6. On an average, these patients lost their income for approximately 10-15 days due to their morbidity, and their attendants also suffered the same. It implies that lower respiratory health-related disease burden in rural population is high. Out of 136 cases studied, 15 cases expired (mortality 11.03%), 4/28 COPD, 8/30 ventilator associated pneumonia, and 3/32 parapneumonic effusions. The total number of patients who left the hospital against medical advice was 17 (PPE, ten, and LRI, seven). File et al. had a comprehensive review of literature to determine the burden of community acquired pneumonia in North American adults. It was conducted to examine the incidence, morbidity and mortality, etiology, antibiotic resistance, and economic impact of CAP in this population. In the United States, there were approximately 4.2 million ambulatory care visits for pneumonia, in 2006. Pneumonia and influenza continue to be a common cause of death in the United States (ranked eighth) and Canada (ranked seventh). In 2005, there were >60,000 deaths due to pneumonia in persons aged >/=15 years in the United States alone. The hospitalization rate for all infectious diseases increased from 1525 hospitalizations per 100,000 persons, in 1998, and to 1667 per 100,000 persons, in 2005. Admission in to an intensive care unit was required in 10% to 20% of patients hospitalized with pneumonia. The mean length of stay for pneumonia was >/=5 days, and the 30-day re-hospitalization rate was as high as 20%. Mortality was highest for CAP patients who were hospitalized; the 30-day mortality rate was as high as 23%. All-cause mortality for CAP patients was as high as 28% within one year. Streptococcus pneumoniae continues to be the most frequently identified pathogen associated with CAP, and pneumococcal resistance to antimicrobials may make treatment more difficult. The economic burden associated with CAP remains substantial at >$17 billion, annually, in the United States. Despite the availability and widespread adherence to recommended treatment guidelines, CAP continues to present a significant burden in adults. [21] Agnihotram et al. in their article titled – “Respiratory disease burden in rural India: A review from multiple data sources” gave the analysis, and stated that poverty and unhealthy environment were strongly related to the respiratory disorders. Bronchitis and asthma recorded as the leading cause, while pneumonia and tuberculosis of the lungs ranked one of the five causes of death in rural India. Asthma and bronchitis prevalence rates in Karnataka, Gujarat, Haryana, Uttar Pradesh, Kerala, and Madhya Pradesh are above the national average. TB prevalence is high in Madhya Pradesh, Uttar Pradesh, and Gujarat, while Tamil Nadu and Maharashtra recorded the lowest prevalence. Outstandingly, pneumonia prevalence is almost five times the national average in Haryana. They reviewed the respiratory disorder burden of rural Indians by utilizing data on survey of cause of death (rural). In low resource settings, these diseases are mainly attributed with exposure to indoor pollution, solid-cooking fuels, poor housing, low nutritional status, and sanitary conditions. The association of respiratory disorders with geographical region may be relevant to the population density, industrial and textile pollutants, and tobacco consumption. [22] The term, “Lower Respiratory Infections”, include a wide variety of clinical conditions like pneumonia, acute bronchitis, acute exacerbation of chronic bronchitis, lung abscess, and emphysema etc. The specimens to be collected vary from sputum, BAL to pleural fluid etc. Microbial etiology of these conditions is equally varied and difficult to establish. Such “wide base” study involving many parameters will always have wide geographic variations. Isolation of Acinetobacter boumannii and Stenotrophomonas maltophilia from a few critically ill ventilated patients in ICU and their sensitivity to higher antibiotics points towards a future trend and warrants strict vigilance. In India, precise studies to estimate respiratory disease burden in rural areas are required for proper area-specific public health intervention. No concrete data exists to show the treatment directed at a specific pathogen is statistically superior to empirical therapy. Without culture and susceptibility data, appropriate empirical therapeutic regimens are harder to devise.
The authors would like to thank the faculty and other staff of ICU and wards of Mamata General Hospital Khammam, A.P., for their valuable support in the collection of samples.
Source of Support: None, Conflict of Interest: None
[Figure 1], [Figure 2]
[Table 1], [Table 2] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||